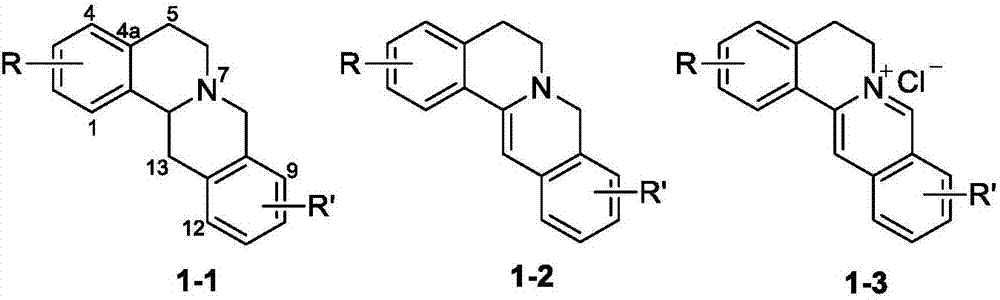
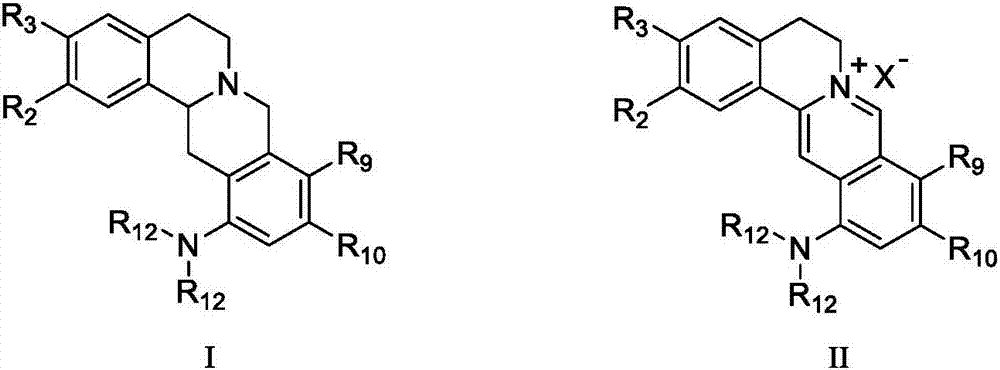
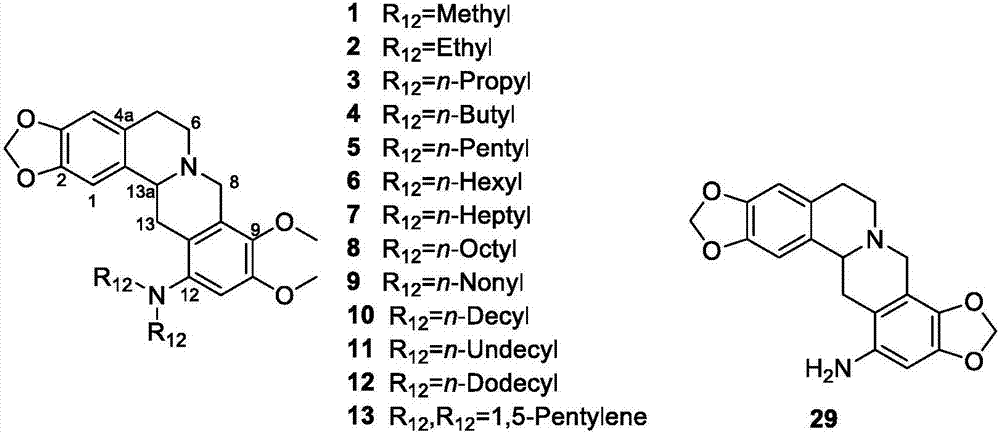
Berberine-based derivative and preparation method thereof, pharmaceutical composition, and antitumor uses of berberine-based derivative and pharmaceutical composition
A technology of berberine and derivatives is applied in the fields of berberine derivatives, their preparation, pharmaceutical compositions and anti-tumor uses, and can solve the problems of poor anti-tumor activity and specificity, affecting druggability, and low bioavailability, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment (1
[0050] The preparation of embodiment (1) compound 1
[0051] Weigh berberine chloride quaternary ammonium salt (20g, 53.84mmol) in reaction flask, add glacial acetic acid (250ml), be placed in ice-water bath, slowly add NaNO 2 (18.6g, 269.57mmol), dropwise into concentrated HNO 3 (30ml), after the dropwise addition was completed, the mixture was stirred in an ice-water bath for another 5 minutes, then heated to reflux at 50° C. for 1 hour, and the reaction was complete. Add water (200ml) to the reaction solution, extract with chloroform / methanol (v / v=10:1), the organic phase obtained is distilled under reduced pressure, removes the organic solvent, and the crude product obtained is subjected to silica gel column chromatography [with chloroform / methanol =20:1 (v / v) is the eluent] purified to obtain 12-nitroberberine chloride, 9.65 g of red solid, yield 43%. 1 H NMR: (400MHz, DMSO-d 6 )δ3.23(t, J=6Hz, 2H, Ar-CH 2 CH 2 N),4.16(s,3H,OCH 3 ),4.28(s,3H,OCH 3 ), 4.96(t, J=6Hz,...
Embodiment
[0052] The preparation of embodiment (2) compound 2
[0053] Weigh 12-aminotetrahydroberberine (250mg, 0.705mmol) in a reaction flask, add dichloromethane (10ml), then add 40% acetaldehyde aqueous solution (313μl, 3.1mmol), triacetoxy borohydrogenation Sodium (747 mg, 3.525 mmol), HOAc (18 drops), stirred at room temperature for 2 h, and the reaction was complete. Add saturated sodium bicarbonate to the reaction solution to adjust the pH to 8, stir at room temperature for 20 min, and then extract with dichloromethane; the dichloromethane extract was washed 3 times with water, once with saturated brine, and dried over anhydrous magnesium sulfate , filtered with suction, and the organic phase was evaporated under reduced pressure, and the crude residue was purified by silica gel column chromatography [with dichloromethane / methanol=80:1 (v / v) as eluent] to obtain compound 2, 277 mg of light brown solid, Yield 96%. 1 H NMR: (400MHz, CDCl 3 )δ0.98(t, J=7.2Hz, 6H, 2×NCH 2 CH 3 ...
Embodiment (7
[0062] The preparation of embodiment (7) compound 7
[0063] Weigh 12-aminotetrahydroberberine (200mg, 0.564mmol) in a reaction flask, add dichloromethane (8ml), then add n-heptanal (189 μl, 1.354mmol), sodium triacetoxyborohydride ( 358mg, 1.692mmol), HOAc (10 drops), the reaction was complete after stirring at room temperature for 2h. Add saturated sodium bicarbonate to the reaction solution to adjust the pH to 8, stir at room temperature for 20 min, and then extract with dichloromethane; the dichloromethane extract was washed 3 times with water, once with saturated brine, and dried over anhydrous magnesium sulfate , suction filtration, the organic phase was evaporated under reduced pressure, and the crude residue was purified by silica gel column chromatography [with dichloromethane / methanol=80:1 (v / v) as eluent] to obtain compound 7, light brown oil 272mg , yield 87.6%. 1 H NMR: (400MHz, CDCl 3 )δ0.85(t, J=6.8Hz, 6H, 2×NCH 2 CH 2 CH 2 CH 2 CH 2 CH 2 CH 3 ),1.23(m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com