Compositions, cured products, prepregs and laminates
A composition and laminate technology, applied in the direction of synthetic resin layered products, layered products, metal layered products, etc., to achieve the effects of achieving adhesion, heat resistance, and easy impregnation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0102] Although an Example and a comparative example are given and this invention is demonstrated concretely, this invention is not limited to these. Unless otherwise specified, "part" means a mass part, and "%" means a mass %. Measuring methods were measured by the following methods, respectively.
[0103] The analysis method and measurement method are shown below.
[0104] (1) Non-volatile components: According to JISK6910 standard (5.6 Non-volatile components). Specifically, when the sample amount was 1 g, the test temperature was 150° C., and the test time was 1 hour, the solid content remaining after distilling off the solvent was used as a nonvolatile content.
[0105] (2) Solubility: Formulated at a predetermined ratio, the case where crystallization was not observed even after being left at room temperature for 1 week and then impacted by stirring was indicated by "○", and the case where uniform dissolution was not possible, or Those that precipitated crystals withi...
Synthetic example 1
[0111]In a separable flask made of glass with a stirring device, a thermometer, a nitrogen gas introduction device, a cooling pipe and a dropping device, add 105 parts of phenol novolak resin (phenolic hydroxyl equivalent (g / eq.) is 105, and the softening point is 130°C), 0.1 parts of p-toluenesulfonic acid, and the temperature was raised to 150°C. While maintaining this temperature, 94 parts of styrene was dripped over 3 hours, and stirring was continued at this temperature for 1 hour. Then, it was dissolved in 500 parts of methyl isobutyl ketone (MIBK), and washed with water five times at 80°C. Then, MIBK was distilled off under reduced pressure to obtain a styrene-modified phenol novolac resin (b-1) represented by the following formula (4). The obtained (b-1) had a phenolic hydroxyl equivalent of 199, a softening point of 110° C., and p (average value) in the formula (4) of 0.9.
[0112] [chemical 5]
[0113]
Synthetic example 2
[0115] In the same device as in Synthesis Example 1, 105 parts of phenol novolak resin (phenolic hydroxyl equivalent: 105, softening point: 67°C) and 0.13 parts of p-toluenesulfonic acid were added, and the temperature was raised to 150°C. While maintaining this temperature, 156 parts of styrene was dripped over 3 hours, and stirring was continued at this temperature for 1 hour further. Then, the same treatment as in Synthesis Example 1 was performed to obtain a styrene-modified phenol novolak resin (b-2). The obtained (b-2) had a phenolic hydroxyl equivalent of 261, a softening point of 75° C., and p of 1.5.
[0116] The description of the symbols used in Examples and Comparative Examples is as follows.
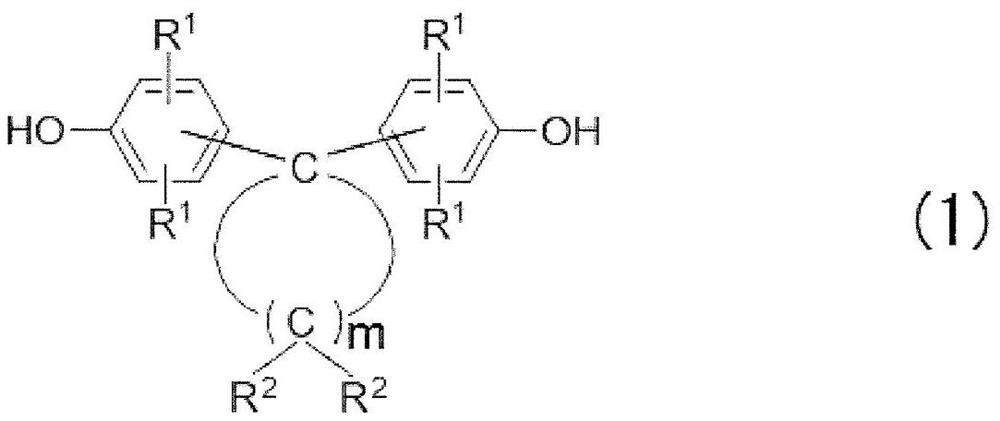
[0117] (1) Bisphenol compound (A):
[0118] BisP-TMC: 4,4'-(3,3,5-trimethylcyclohexylene)bisphenol (manufactured by Honshu Chemical Industry Co., Ltd., BisP-TMC, phenolic hydroxyl equivalent: 155, melting point: 206°C)
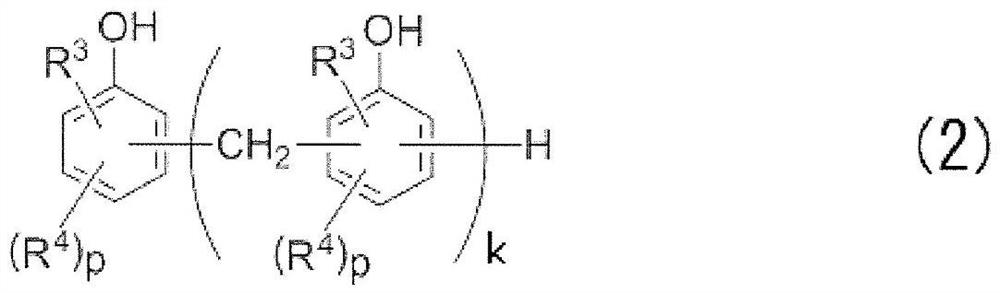
[0119] (2) Phenolic compound (B):
[0120] (b-1): ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com