GM hybridoma, monoclonal antibody, kit, preparation method and application thereof
一种杂交瘤细胞、单克隆抗体的技术,应用在生物化学设备和方法、化学仪器和方法、抗真菌/藻类/地衣免疫球蛋白等方向,能够解决检测步骤繁琐、单克隆抗体制备成本高、不利于试剂盒推广和普及等问题,达到准确可靠检验结果、减少经验性抗真菌治疗、专一性高的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
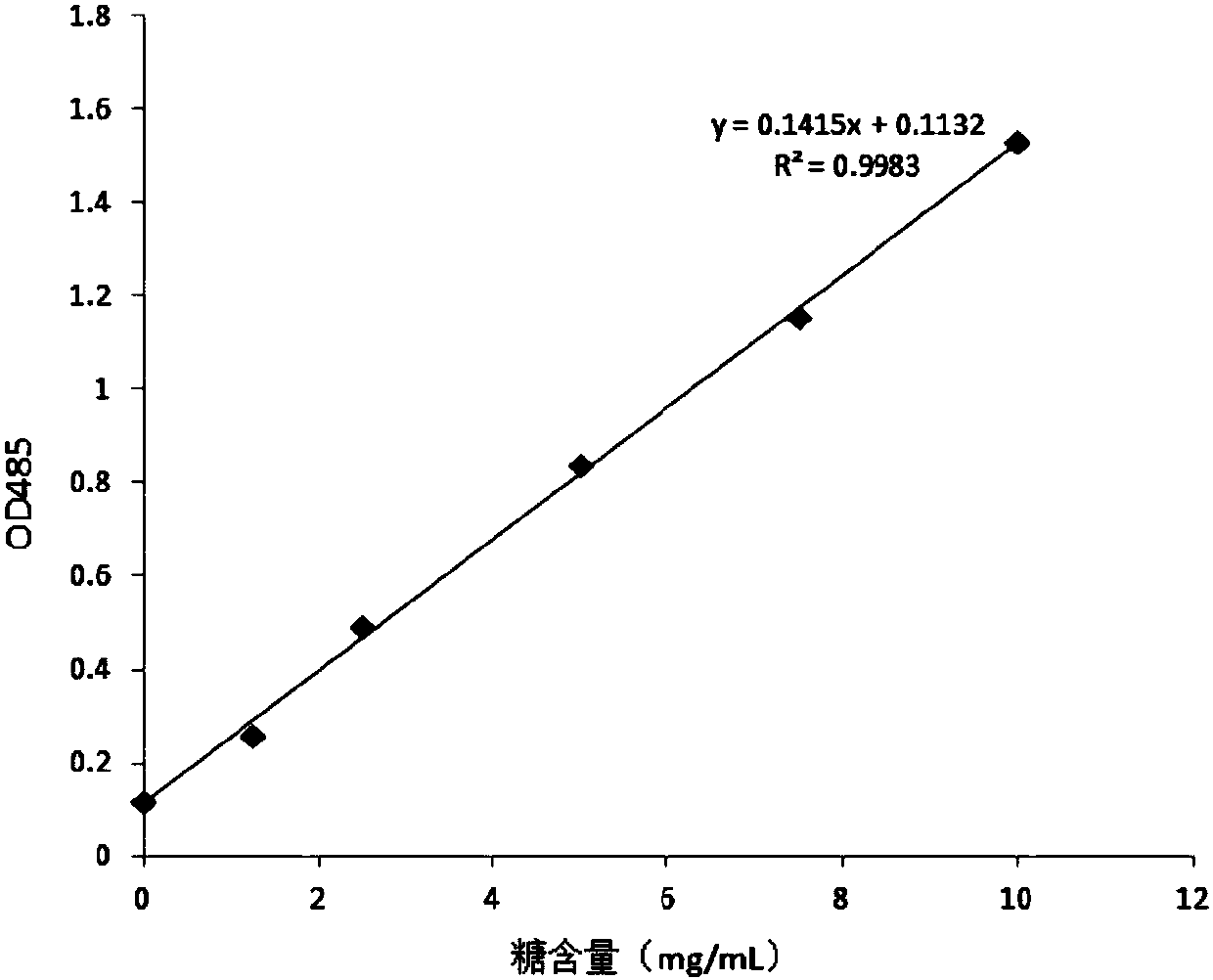
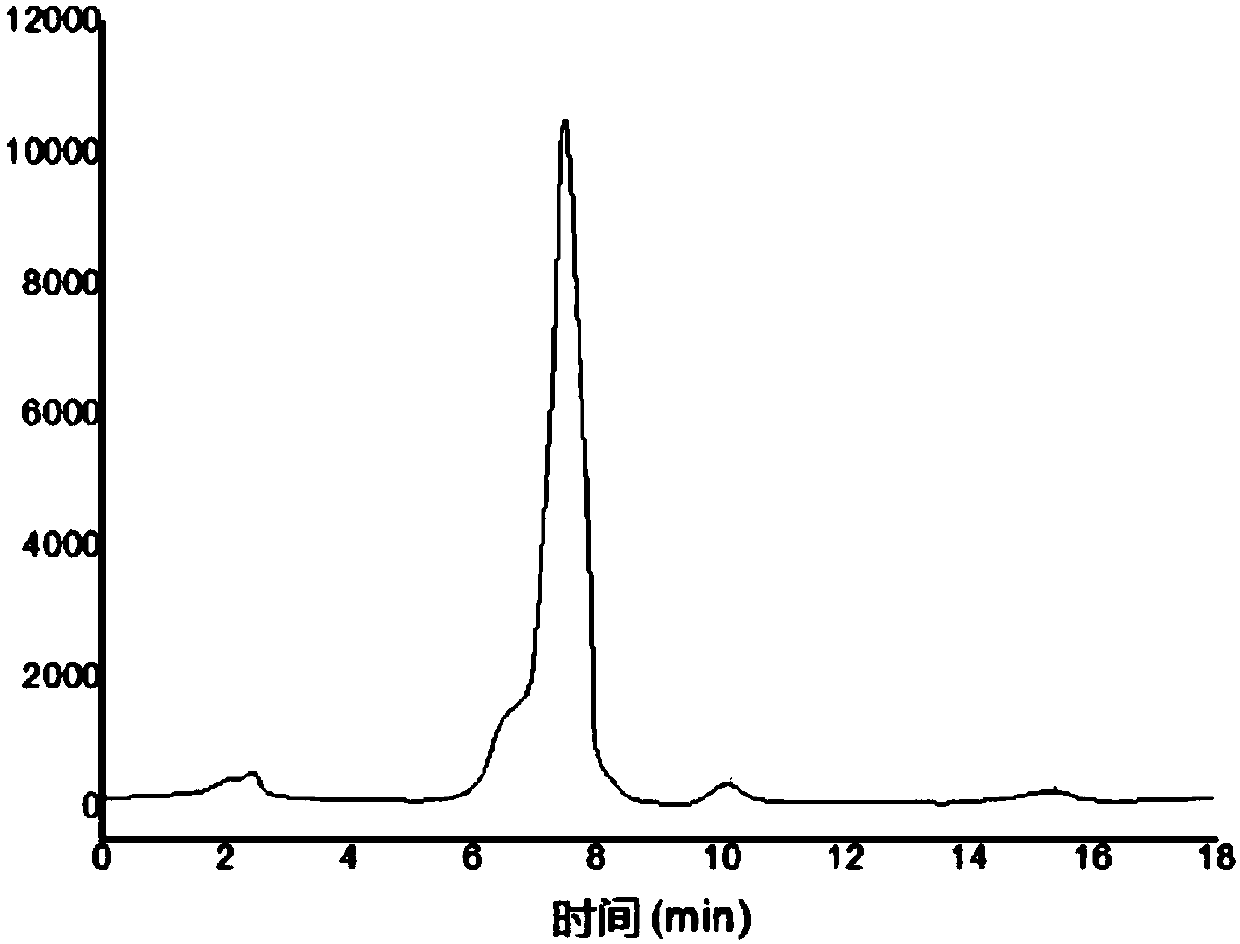
[0038] The preparation of embodiment 1 Aspergillus galactomannan (GM) antigen
[0039] Aspergillus is used to prepare the GM antigen. The Aspergillus strain used in the present invention is purchased from the American Type Culture Collection (ATCC), and the preservation number is ATCC 1022.
[0040] Cultivate Aspergillus with solid medium until the medium is covered with green spores; filter to remove mycelium, inactivate the bacteria and spores; after centrifugation, collect the spores and wash; the spores are broken and filtered to remove spore fragments; the filtrate is subjected to alcohol precipitation 1. After washing, the GM antigen crude extract is obtained; the GM antigen crude extract is decolorized and ultrafiltered to obtain the GM antigen.
[0041] Wherein, the solid medium is selected from PDA medium, Sabouraud medium or Zabuba medium; preferably PDA medium. Specific steps are as follows:
[0042] 1. Preparation of GM crude product
[0043] Prepare PDA solid m...
Embodiment 2
[0064] Example 2 Modification of GM antigen
[0065] It is well known that molecular size can affect the formation of immunogenicity of substances, and the molecular weight of an effective immunogen is mostly above 10kD; the larger the molecular weight, the stronger the immunogenicity. This may be due to the fact that macromolecular substances are easy to form colloids in aqueous solution, stay in the body for a long time, and have more opportunities to contact immune cells, which is conducive to stimulating the body to produce an immune response. In addition, the chemical structure of macromolecular substances is relatively complex, and the types and quantities of effective antigen genes contained are relatively large.
[0066] The GM antigen obtained in Example 1 has a small molecular weight and poor immunogenicity, and needs to be coupled with related macromolecular substances to improve its immunogenicity. The macromolecules are one or more of latex microspheres, KLH (key...
Embodiment 3
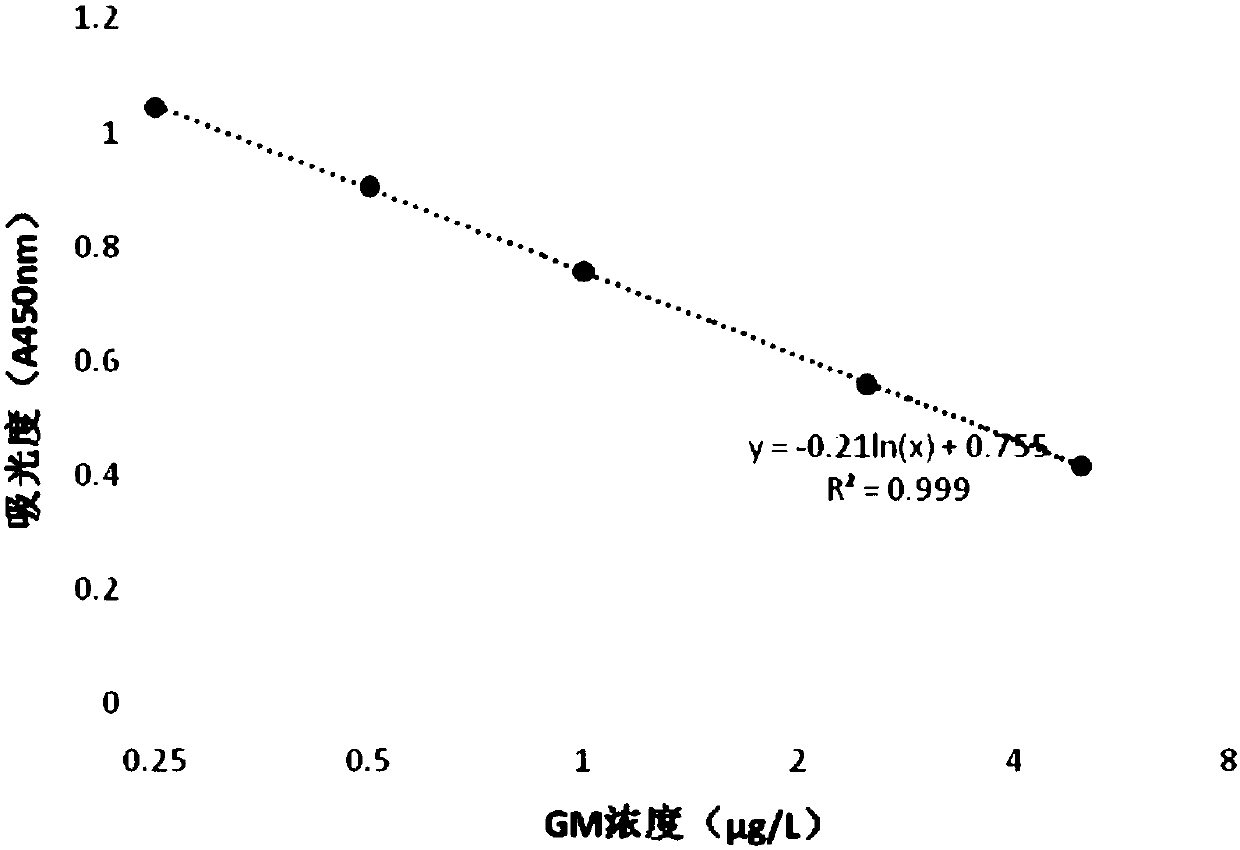
[0076] The preparation of embodiment 3 anti-GM antigen monoclonal antibodies
[0077] 1. Preparation of monoclonal antibody against GM antigen
[0078] 1. Immunization of animals
[0079] Animals were immunized with GM antigen. Among them, the immunization can be performed by subcutaneous injection, foot pad injection, intrasplenic injection, intravenous injection or intraperitoneal injection; the animals can be rats, mice, guinea pigs, rabbits, chickens, sheep, horses, pigs or donkeys. In this embodiment, rabbits are used as immunized animals, and the specific steps are as follows:
[0080] Mix equal volumes of GM antigen and Freund's complete adjuvant to an appropriate volume, fully emulsify and inject New Zealand big-eared rabbits subcutaneously at multiple points, and the immunization dose of each rabbit is controlled at 0.01-0.1mg. Ear blood was collected 3 days before immunization, and serum was separated as a negative control. Immunize once every 2 weeks after the f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com