Synthesis method of amoxicillin artificial antigen
A technology of amoxicillin and artificial antigen, which is applied in the field of biochemical industry, can solve the problems of low product synthesis rate, many by-products, reducing antigen immunogenicity, etc., and achieve the effects of enhancing immune effect, increasing molecular weight, and enhancing immunogenicity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
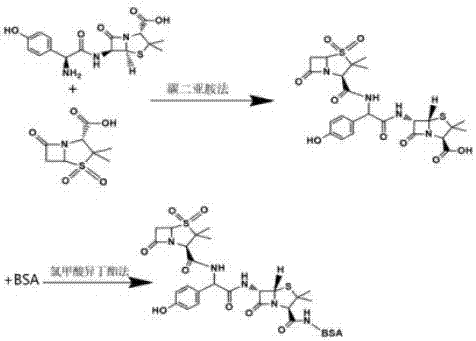
[0029] A kind of synthetic method of amoxicillin artificial antigen, comprises the steps:
[0030] (1) Synthesis of haptens
[0031] Amoxicillin and sulbactam were coupled by the carbodiimide method at a molar ratio of 1:1.5. First, the sulbactam was dissolved with 2-morpholineethanesulfonic acid buffer, and then the sulbactam was mixed with carbon diimide The imine was added to the carbodiimide solution at a molar ratio of 1:4, and after reacting for 2 hours under magnetic stirring, the reaction solution was freeze-dried to obtain a sulbactam-coupled amoxicillin hapten solid.
[0032] (2) Synthesis of complete antigen
[0033] Dissolve 2 mg of the hapten obtained in step (1) with 1 mL of N-N dimethylformamide, add 20 μL of tri-n-butylamine, react at 4°C for 20 min, then add 35 μL of isobutyl chloroformate to continue the reaction for 2 h, and dissolve it Add dropwise at a molar ratio of 50:1 to bovine serum albumin BSA dissolved in 0.01M pH7.2 hydroxyethylpiperazine ethanes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com