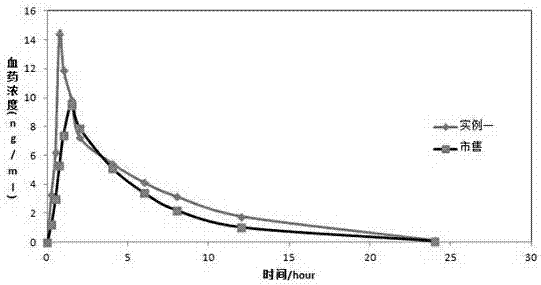
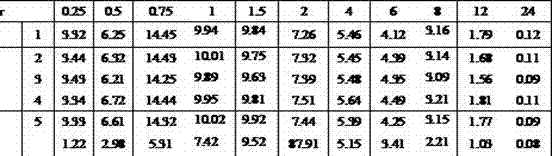
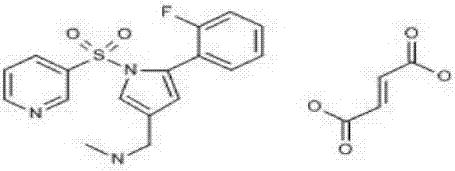
Vonoprazan fumarate solid dispersions and preparation method thereof
A technology for vonoprazan fumarate and solid dispersion, which is applied in the field of vonoprazan fumarate solid dispersion and tablets thereof, can solve the problems of poor water solubility, low bioavailability and the like, and achieves reduction in dosage, The preparation process is simple and the effect of improving the dissolution rate in vitro
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: 1000 pieces of specifications
[0034] prescription:
[0035] Ingredients Dosage per tablet (mg)
[0036] Vonoprazan Fumarate 13
[0037] Polyvinylpyrrolidone K30 30
[0038] Mannitol 170
[0039] Sodium starch glycolate 7
[0041] Preparation method:
[0042] (1) Take 1300mg of vonoprazan fumarate, add it to 100ml of organic solvent, dissolve it by ultrasonic, weigh PVP K30, add 30000mg to the above organic solvent, and dissolve it by ultrasonic. Place the organic solution on a rotary evaporator at 55-60°C to evaporate the organic solvent under reduced pressure, dry the product in a vacuum oven for 24 hours, pulverize, grind and sieve to obtain a solid dispersion of vonoprazan fumarate.
[0043] (2) Weigh the prescribed amount of mannitol, sodium carboxymethyl starch, and magnesium stearate, mix them evenly, and then compress into tablets.
Embodiment 2
[0044] Embodiment 2: 1000 pieces of specifications
[0045] prescription:
[0046] Ingredients Dosage per tablet (mg)
[0047] Vonoprazan Fumarate 13
[0048] Polyvinylpyrrolidone K30 33
[0049] Lactose 170
[0050] Croscarmellose Sodium 7
[0052] Preparation method:
[0053] (1) Take 13000mg of vonoprazan fumarate, add it to 200ml of organic solvent, dissolve it by ultrasonic, weigh PVP K30, add 33000mg to the above organic solvent, and dissolve it by ultrasonic. Place the organic solution on a rotary evaporator at 55-60°C to evaporate the organic solvent under reduced pressure, dry the product in a vacuum oven for 24 hours, pulverize, grind and sieve to obtain a solid dispersion of vonoprazan fumarate.
[0054] (2) Weigh the prescribed amount of mannitol, lactose, croscarmellose sodium, and magnesium stearate, mix well, and then compress into tablets.
Embodiment 3
[0055] Embodiment 3: 1000 pieces of specifications
[0056] prescription:
[0057] Ingredients Dosage per tablet (mg)
[0058] Vonoprazan Fumarate 13
[0059] Poloxamer 30
[0060] Microcrystalline Cellulose 170
[0061] Povidone 7
[0063] Preparation method:
[0064] (1) Take 13,000 mg of vonorazan fumarate, add it to 100 ml of organic solvent, and dissolve it by ultrasonication, weigh 30,000 mg of poloxamer, add it to the above organic solvent, and dissolve it by ultrasonication. Place the organic solution on a rotary evaporator at 55-60°C to evaporate the organic solvent under reduced pressure, dry the product in a vacuum oven for 24 hours, pulverize, grind and sieve to obtain a solid dispersion of vonoprazan fumarate.
[0065] (2) Weigh the prescribed amount of mannitol, microcrystalline cellulose, povidone, talcum powder, mix well, and then press
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com