Crystal of aldactone and preparation method thereof
A technology of spironolactone and crystal, applied in the new crystal form of spironolactone and the field of preparation thereof, can solve problems such as poor crystal form I stability, poor product stability, inability to practical application, etc., achieves high reproducibility, mild conditions, and is conducive to large-scale The effect of industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
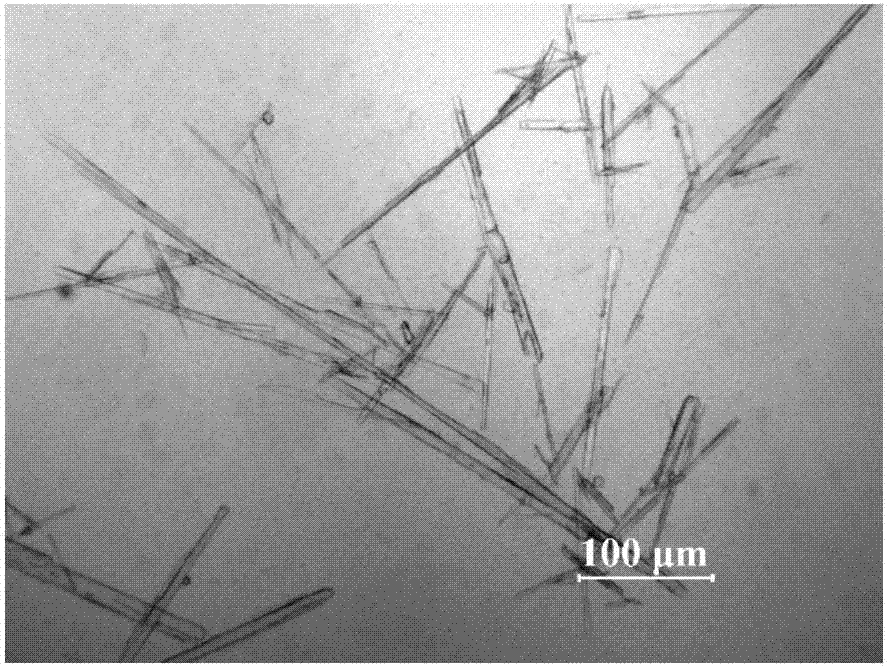
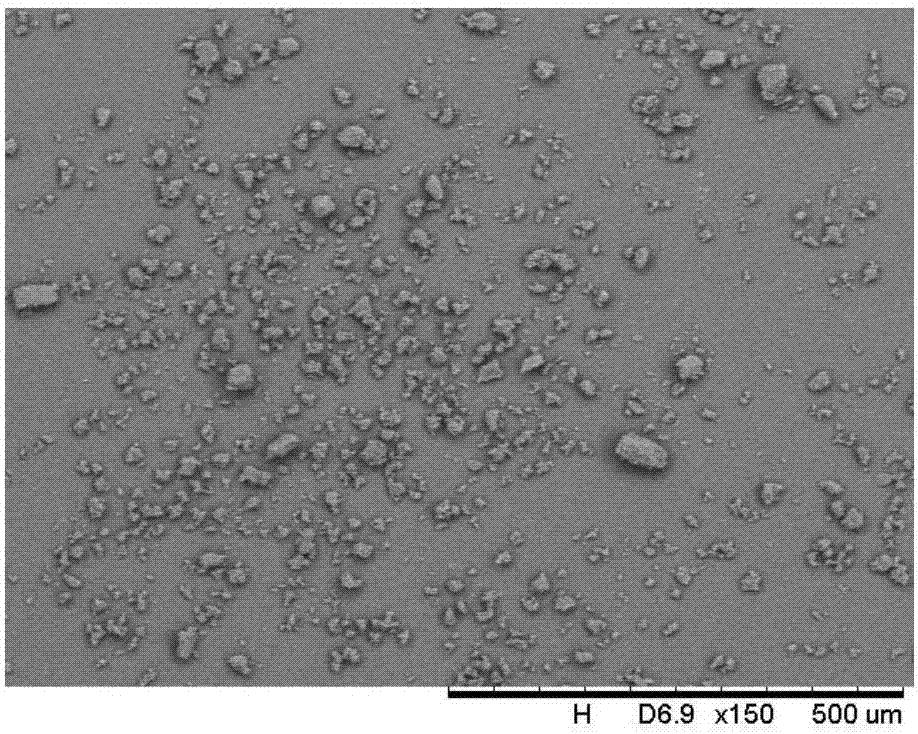
[0037]At a constant temperature of 30°C, add 20.00g of spironolactone solid into 20ml of dichloromethane to form a suspension; place the suspension in an ultrasonic system for 30 minutes of ultrasonication, the ultrasonic temperature is 30°C, the frequency is 20KHz, and the power is 70W / mL, until the spironolactone is completely Dissolve; add 30ml of petroleum ether dropwise to the solution at a rate of 1.2ml / min; cool the suspension to 0°C at a cooling rate of 0.05°C / min, and grow crystals for 5h; vacuum filter the obtained suspension, wash, that is A wet product was obtained; the obtained product was dried at 80° C. under vacuum for 12 hours to constant weight to obtain a new crystal form of spironolactone.
[0038] The X-ray powder diffraction pattern of the product is at diffraction angle 2θ=8.2, 9.8, 10.0, 12.8, 13.6, 13.8, 15.4, 16.0, 16.5, 16.8, 18.1, 18.8, 21.3, 22.0, 24.0, 25.4, 26.3, 27.2, 29.9 , There is a characteristic peak at 35.9 degrees. The appearance of the ...
Embodiment 2
[0040] At a constant temperature of 45°C, add 6.01g of spironolactone solids into 20ml of n-propanol to form a suspension; place the suspension in an ultrasonic system for 10 minutes of ultrasonication, the ultrasonic temperature is 30°C, the frequency is 40KHz, and the power is 50W, until the spironolactone is completely dissolved; Add 10ml of methanol dropwise to the solution at a rate of 0.15ml / min; cool the suspension to 4°C at a cooling rate of 1.0°C / min, and grow crystals for 1 hour; vacuum filter the obtained suspension and wash to obtain a wet product ; The resulting product was dried at 120° C. under vacuum for 4 hours to constant weight to obtain a new crystal form of spironolactone.
[0041] The X-ray powder diffraction pattern of the product is at diffraction angle 2θ=8.2, 9.8, 10.0, 12.9, 13.6, 13.8, 15.4, 16.0, 16.5, 16.8, 18.2, 18.9, 21.2, 22.0, 24.0, 25.3, 26.4, 27.1, 29.9 , There is a characteristic peak at 36.0 degrees. The appearance of the product is block...
Embodiment 3
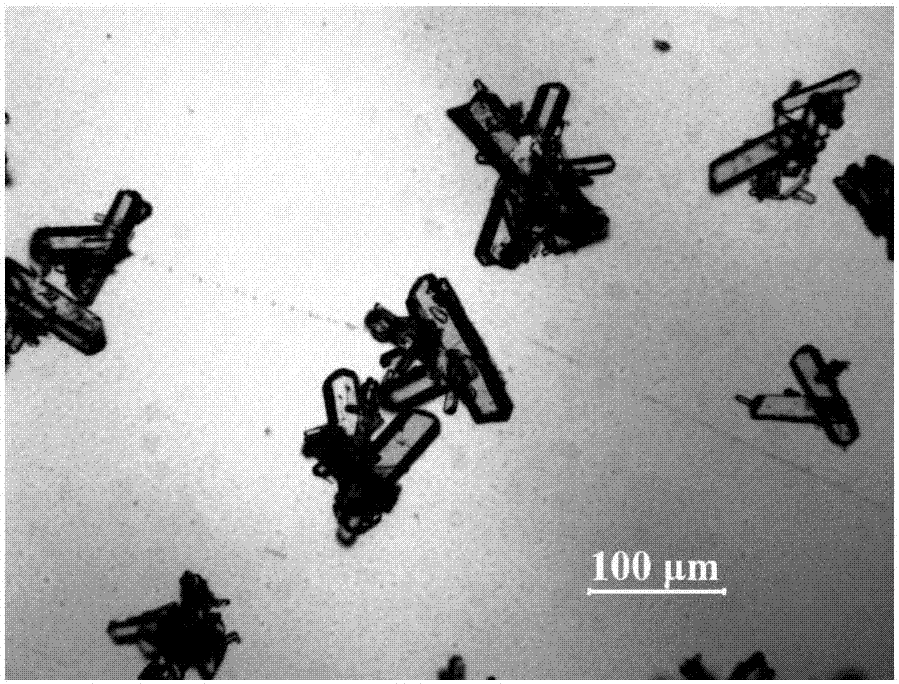
[0043] At a constant temperature of 50°C, add 4.50g of spironolactone solid into 15ml of n-butanol to form a suspension; place the suspension in an ultrasonic system for 30 minutes of ultrasonication, the ultrasonic temperature is 30°C, the frequency is 60KHz, and the power is 70W, until the spironolactone is completely dissolved; Add 15ml of distilled water dropwise to the solution at a rate of 0.3ml / min; cool the suspension to 10°C at a cooling rate of 0.8°C / min, and grow crystals for 4 hours; vacuum filter the resulting suspension and wash to obtain a wet product ; The resulting product was dried at 100° C. under vacuum for 12 hours to constant weight to obtain a new crystal form of spironolactone.
[0044] The X-ray powder diffraction pattern of the product is at diffraction angle 2θ=8.3, 9.9, 10.1, 12.9, 13.8, 13.8, 15.4, 16.0, 16.5, 16.8, 18.1, 18.8, 21.3, 22.2, 24.2, 25.4, 26.5, 27.4, 29.9 , There is a characteristic peak at 35.7 degrees. The appearance of the product ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com