Compound with motoneuron growth promoting effect, preparation method and use thereof
A technology of motor neurons and compounds, applied in the field of natural medicine and drug therapy, to achieve the effect of promoting functional recovery and promoting the growth of motor neuron protrusions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: compound synthesis
[0028] Oleanolic acid (1.1 mmole) and nicotinic acid (1.3 eq) were dissolved in anhydrous dichloromethane (30 ml), under the catalysis of pyridine (0.5 g) and 4-dimethylaminopyridine (0.5 g), at room temperature React overnight at 25-30°C, filter, pass through a silica gel chromatographic column (the elution solvent used is ethyl acetate and petroleum ether with a volume ratio (3-9): 7), rotary evaporation, and dry to obtain the white solid target compound. Through liquid chromatography mass spectrometry and NMR analysis, the newly prepared white solid target compound is a conjugate with the structure shown in formula (I):
[0029]
Embodiment 2
[0030] Example 2: Drug function verification at the cellular level
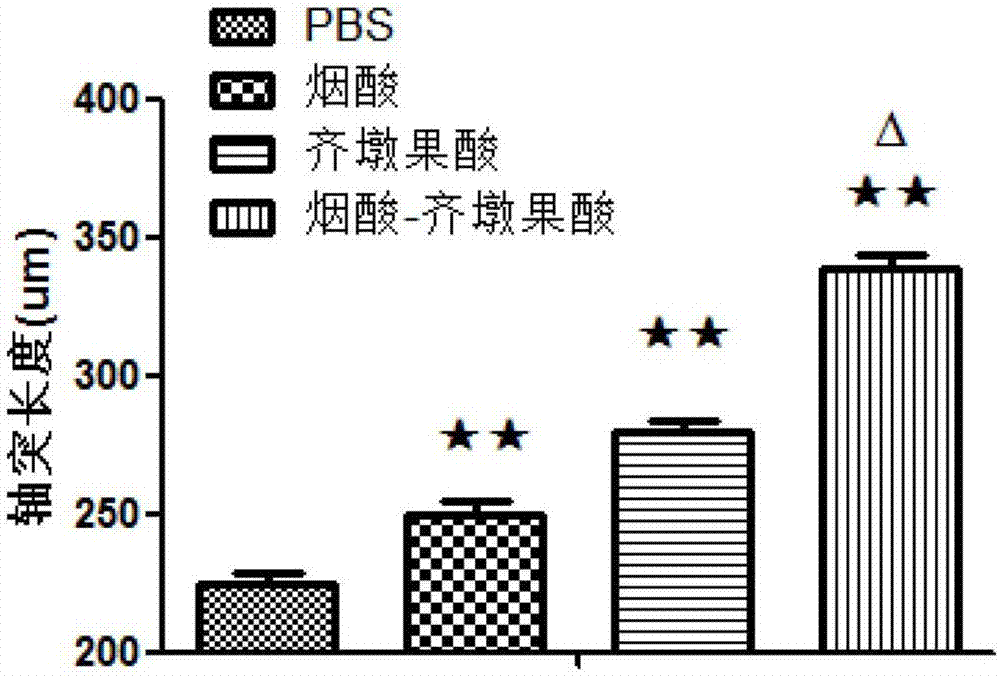
[0031] After the drugs (PSB, nicotinic acid, oleanolic acid and the target compound obtained in Example 1) were applied to the primary cultured motor neurons for 24 hours, the axonal growth of the neurons was observed under an inverted microscope, with 3 wells in each group. A number of fields of view were randomly selected in each well to take pictures, and the statistical process length was measured by IPP software. The values of each group were compared with those of the PBS control group to evaluate the effect of drugs on the process length of motor neurons. Next, the lengths of motor neuron axons were counted at different concentrations, and it was found that the five drugs can all promote the growth of neuron processes at a certain concentration, and there are significant differences compared with PBS ( figure 1 ).
Embodiment 3
[0032] Embodiment 3 animal level is carried out drug function verification
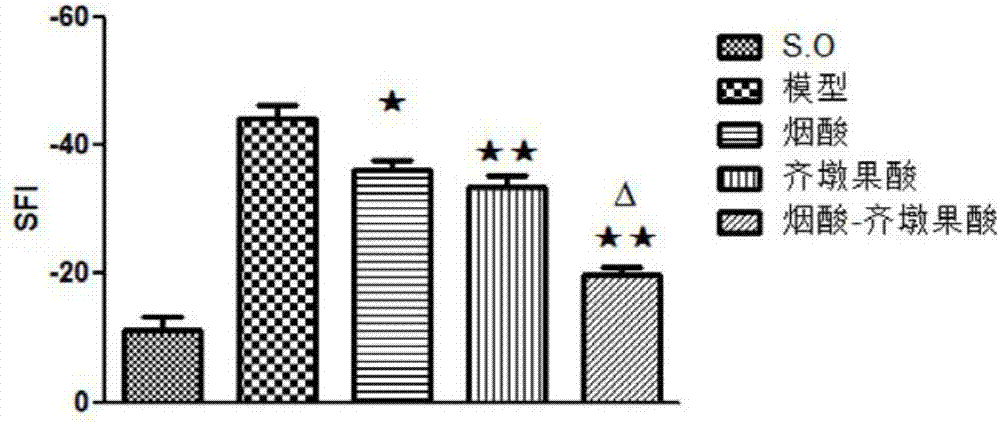
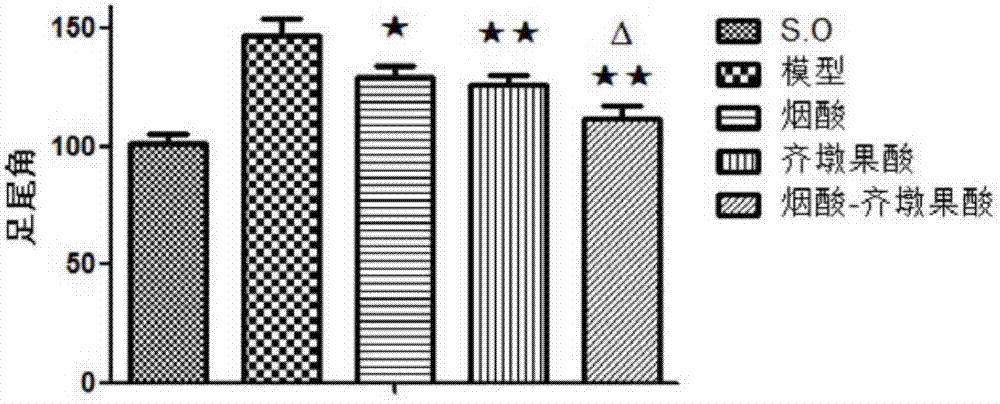
[0033] Wistar rats were subjected to sciatic nerve and femoral nerve clamping operations, and drugs (niacin, oleanolic acid and the target compound obtained in Example 1) were administered by intraperitoneal injection for 28 days after the operation. By detecting the exercise ability and histological changes of the experimental animals before the administration, 14 days after the administration, and 28 days after the administration, the effect of the drug on the recovery of the exercise ability of the experimental animals was evaluated. The experimental animals of different groups were counted respectively in behavioral detection: SFI value ( figure 2 ), foot tail angle ( image 3 ), plantar angle ( Figure 4 ), outstretched leg length ratio ( Figure 5 ) and other detection indicators, it is confirmed that the drugs can promote the recovery of the exercise ability of experimental animals at a cer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com