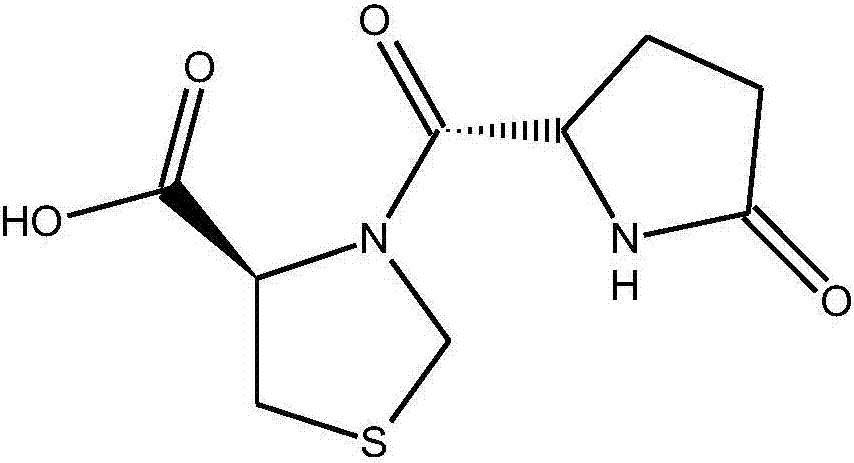
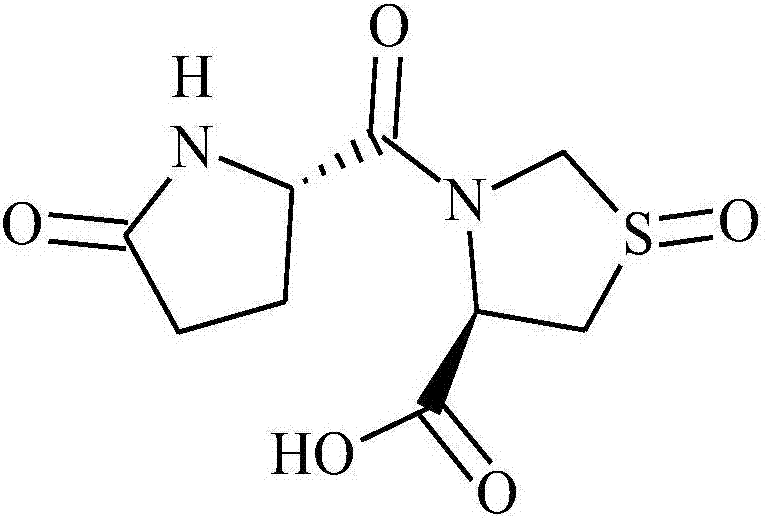
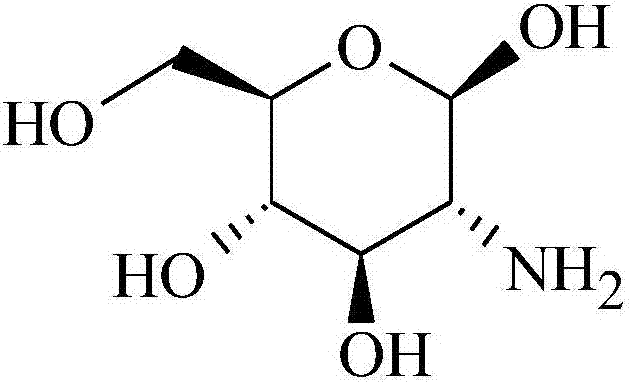
Pidotimod oral solution and preparation method thereof
An oral solution and technology of pidotimod, applied in the field of pharmaceutical composition containing pidotimod and its preparation, can solve the problems of poor stability, intensified molecular movement, difficulty in ensuring safety, etc., and achieve improved solubility , High product stability, the effect of improving product quality and safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] formula:
[0041]
[0042]
[0043] Preparation Process:
[0044] 1) Preparation of the drug-containing solution: Dissolve the prescribed amount of glucosamine in 600ml of water at 40°C, add the prescribed amount of pidotimod, sorbitol, disodium edetate, and grape seed proanthocyanidin extract and stir to dissolve, Cool down to room temperature;
[0045] 2) Preparation of sucralose and preservative solution: dissolve the prescribed amount of sucralose, sodium methylparaben and sodium propylparaben in 50ml of water at room temperature;
[0046] 3) Add 2) into 1), stir evenly, add cherry essence, and dilute to 700ml with purified water to obtain the product.
Embodiment 2
[0048] formula:
[0049]
[0050] Preparation Process:
[0051] 1) Preparation of the drug-containing solution: Dissolve the prescribed amount of glucosamine in 600ml of water at 40°C, add the prescribed amount of pidotimod, sorbitol, disodium edetate, and grape seed proanthocyanidin extract and stir to dissolve, Cool down to room temperature;
[0052] 2) Preparation of sucralose and preservative solution: dissolve the prescribed amount of sucralose, sodium methylparaben and sodium propylparaben in 50ml of water at room temperature;
[0053] 3) Add 2) into 1), stir evenly, add cherry essence, and dilute to 700ml with purified water to obtain the product.
Embodiment 3
[0055] formula:
[0056]
[0057]
[0058] Preparation Process:
[0059] 1) Preparation of the drug-containing solution: Dissolve the prescribed amount of glucosamine in 600ml of water at 40°C, add the prescribed amount of pidotimod, sorbitol, disodium edetate, and grape seed proanthocyanidin extract and stir to dissolve, Cool down to room temperature;
[0060] 2) Preparation of sucralose and preservative solution: dissolve the prescribed amount of sucralose, sodium methylparaben and sodium propylparaben in 50ml of water at room temperature;
[0061] 3) Add 2) into 1), stir evenly, add cherry essence, and dilute to 700ml with purified water to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com