Tenvermectin derivatives and anti-parasitic function of tenvermectin derivatives
A technology of asvermectin and its use, which is applied in the field of asvermectin derivatives and its anti-parasites, and can solve problems such as aquatic biological hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
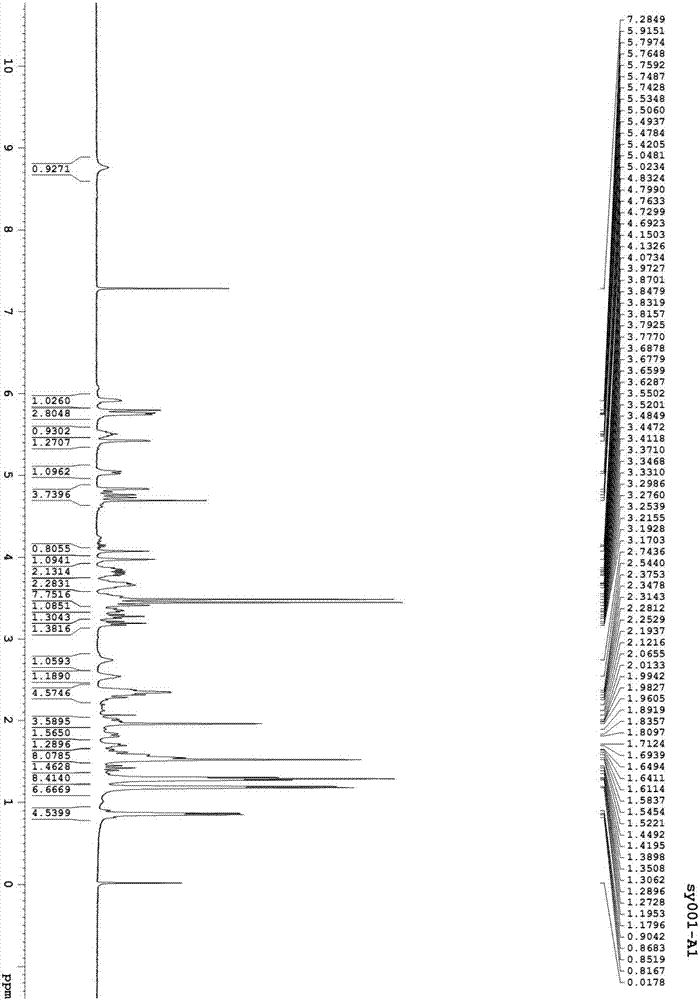
[0072] Preparation of Asvermectin A1 and Asvermectin B1
[0073] (1) oxidation reaction
[0074]
[0075] Put 60ml of acetone into a dry and clean 250ml four-neck flask, add 5g of asvermectin A to electromagnetic stirring and stir to dissolve, cool in an ice-water bath to 10-15°C, add 12g of active manganese dioxide, stir for 10 minutes, and then add active Manganese dioxide 4g, remove the ice-water bath, stir and react at room temperature for 2 hours, sample HPLC, the residue of asvermectin A is less than 2%, add methanol 60ml and stir for 10 minutes, filter with diatomaceous earth filter, wash the filter cake with appropriate amount of methanol , and the filtrate was concentrated to dryness to obtain 4.89 g of asvermectin A oxide as a foamy solid. Dissolve 4.89g of the above-mentioned bubbly solid product in 50ml mobile phase, and put it on the preparative column (instrument: Beijing Innovation Tongheng Technology Co., Ltd.; column: DAC100 preparative column, C18 filler; m...
Embodiment 2
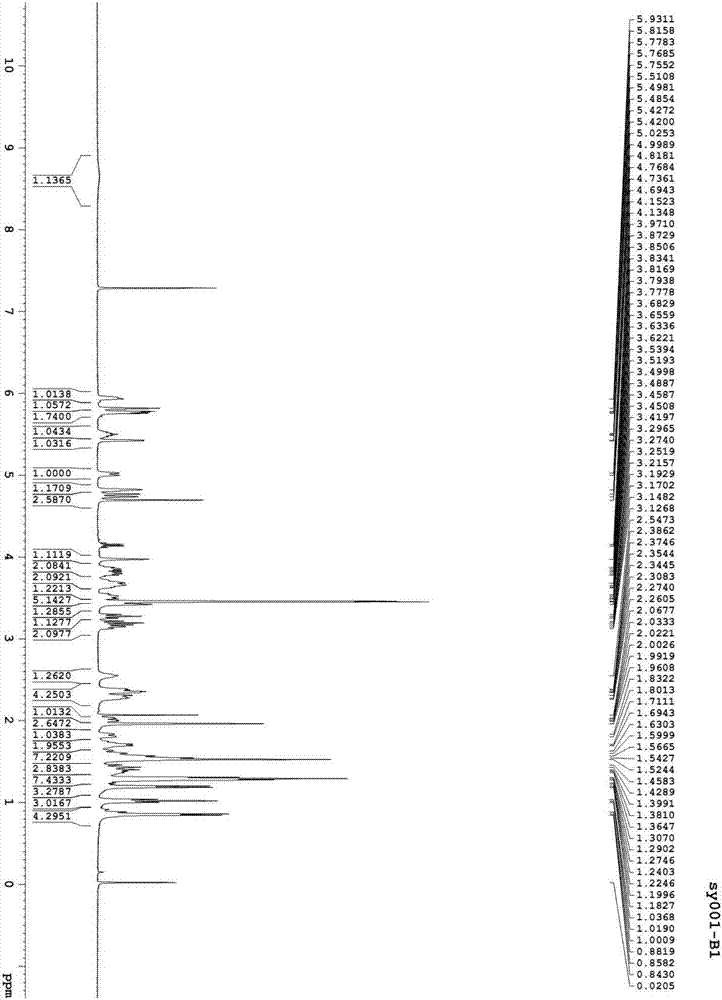
[0082] Preparation of asvermectin A2 and asvermectin B2
[0083]
[0084] In a dry and clean 250ml four-neck flask, drop 3.0g asvermectin A1 (gained in Example 1), 30ml of methanol, stir and dissolve with electromagnetic stirring, add 4ml of 10% sulfuric acid aqueous solution, stir and react at room temperature for 24 hours, and dissolve with 5% carbonic acid Sodium hydrogen solution to adjust the pH to 6-7, add 50ml of water and 50ml of ethyl acetate and stir for 10 minutes, let stand to separate layers, dry the organic phase, dry over anhydrous sodium sulfate, filter, concentrate the filtrate to dryness, and obtain 2.79g of a foamy solid product . Dissolve 2.79g of the above-mentioned bubbly solid product in 40ml mobile phase, and put it on the preparative column (instrument: Beijing Innovation Tongheng Technology Co., Ltd.; column: DAC100 preparative column, C18 filler; mobile phase: acetonitrile: water=8:2; detection Wavelength: 243nm), loading about 14ml each time, co...
Embodiment 3
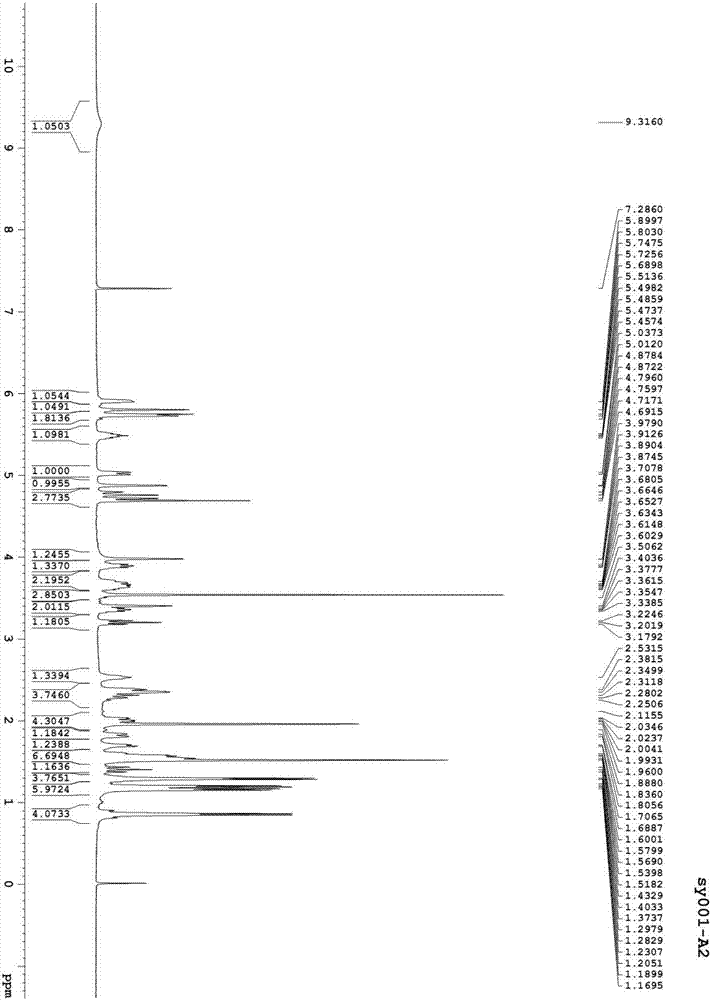
[0088] Preparation of asvermectin A3 and asvermectin B3
[0089]
[0090] In a dry and clean 250ml four-necked flask, drop into 3.0g asvermectin B1 (gained in Example 1), 30ml of methanol, electromagnetic stirring and stirring for dissolving, add 6ml of 10% sulfuric acid aqueous solution, 36 ℃ of stirring reaction 18 hours, then use 5 % sodium bicarbonate solution to adjust the pH to 6-7, add 50ml of water and 50ml of ethyl acetate and stir for 10 minutes, let stand to separate layers, dry the organic phase, dry over anhydrous sodium sulfate, filter, and concentrate the filtrate to dryness to obtain a foamy solid product 2.31 g. Dissolve 2.31g of the above-mentioned bubbly solid product in 40ml mobile phase, and put it on the preparative column (instrument: Beijing Innovation Tongheng Technology Co., Ltd.; column: DAC100 preparative column, C18 filler; mobile phase: acetonitrile: water=85:15; detection Wavelength: 243nm), loading about 13ml each time, collecting the main p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com