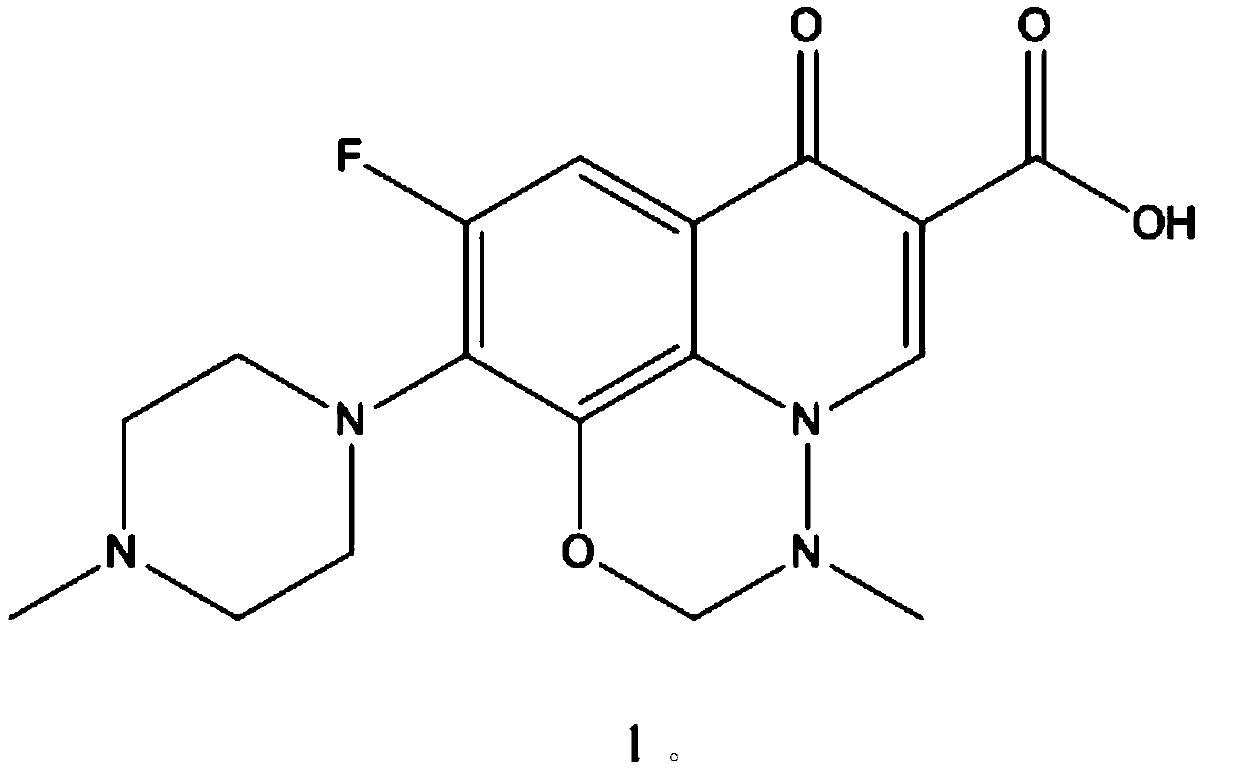
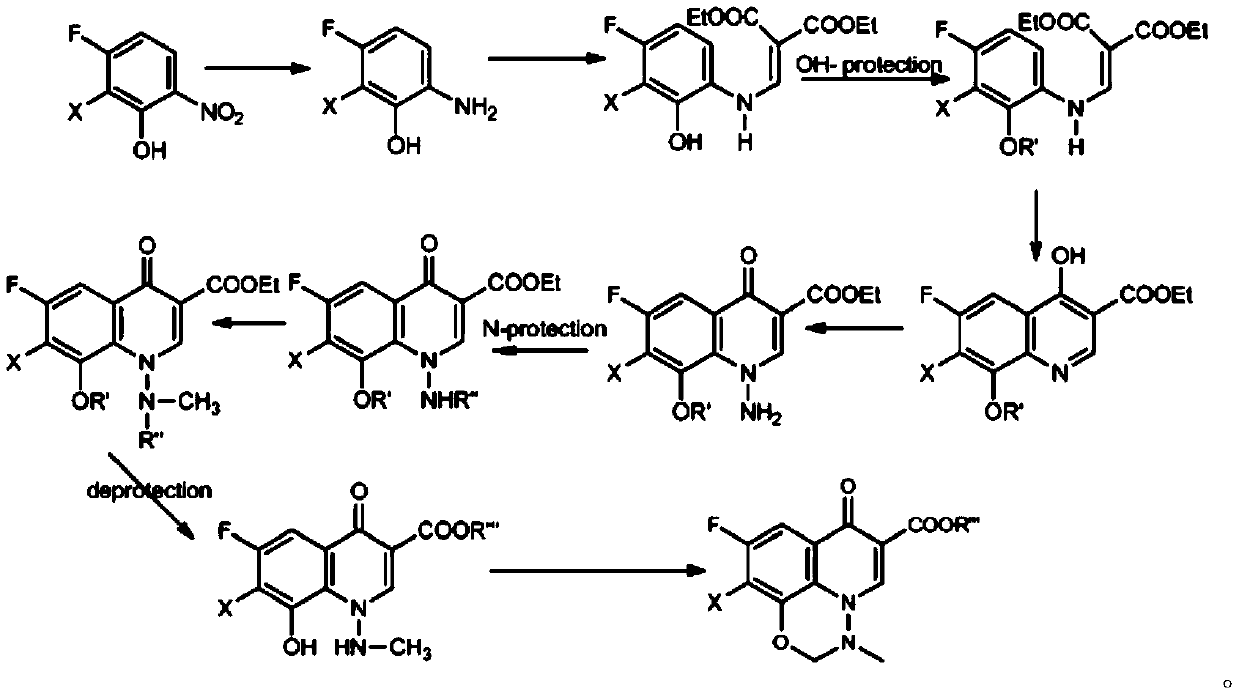
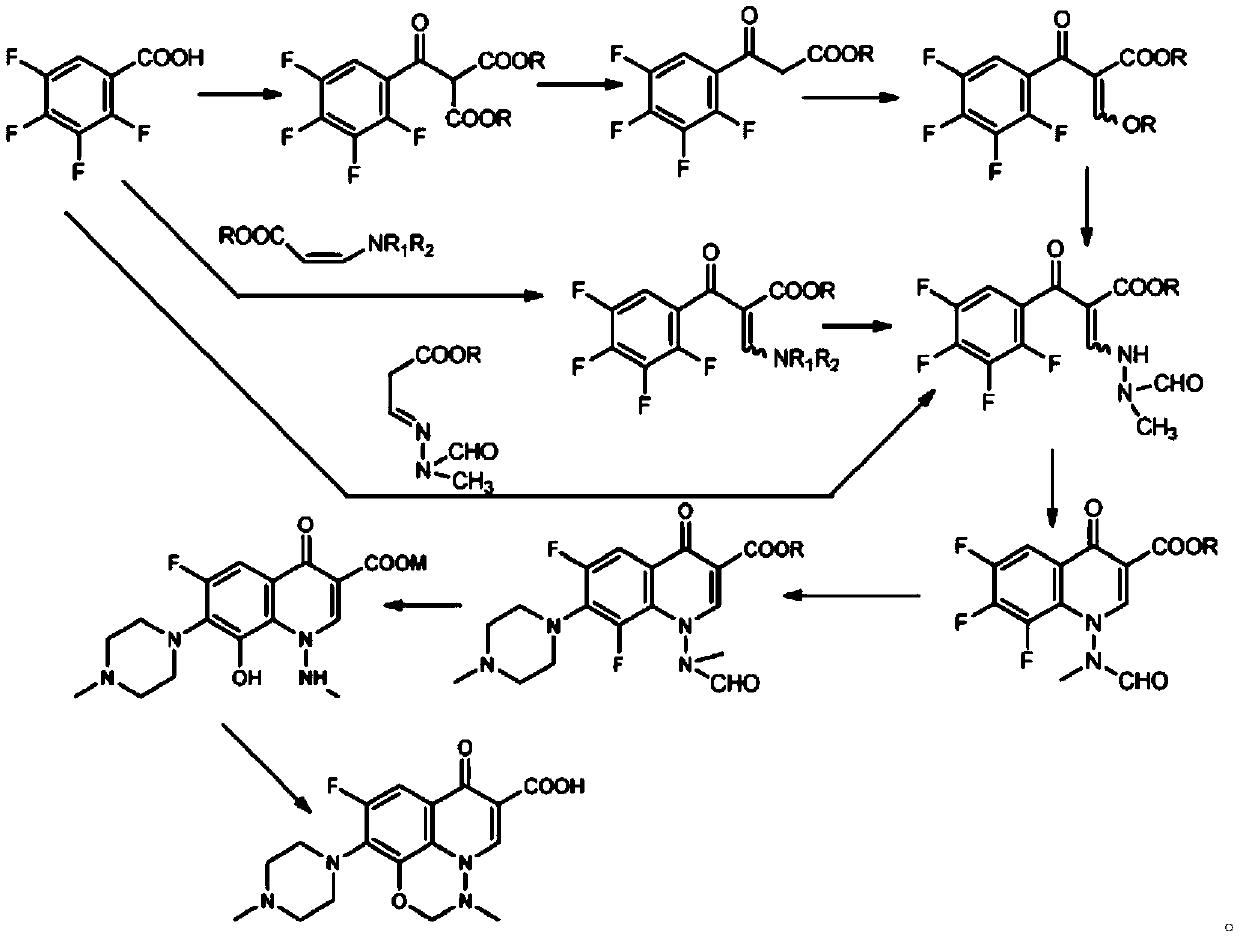
A kind of synthetic method of marbofloxacin
A technology of marbofloxacin and a synthesis method, applied in the direction of organic chemistry and the like, can solve the problems of unfriendly environment, expensive reagents, low yield and the like, and achieve the effects of low price, improved one-time yield, and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] In the four-necked flask, add 30.0g (0.1336mol) of 2,4,5-trifluoro-3-methoxy-benzoyl chloride (compound (2)) and 20.2g (0.2004mol) of triethylamine, add After completion, control at 47°C, add 21.2g (0.1480mol) of N,N-dimethylaminoethyl acrylate (compound 3) dropwise, after dropwise addition, control the internal temperature at 89°C to keep the reaction, keep warm for 1h, finish keeping warm, cool down to At 30°C, add 100g of ethyl acetate to raise the temperature and reflux, cool to 0°C, perform crystal suction filtration, and dry the filter cake to obtain compound (4), weighing 42.3g (95.6% yield, 98.3% chromatographic content).
Embodiment 2
[0042] In the four-neck flask, add 30.0g (0.1336mol) of 2,4,5-trifluoro-3-methoxy-benzoyl chloride (compound (2)) and 29.9g (0.1400mol) of tri-n-butylamine, After the addition, control the temperature at 11°C, add 22.9g (0.1600mol) of N,N-dimethylaminoethyl acrylate (compound 3) dropwise, after the dropwise addition, control the internal temperature at 53°C to keep the reaction, keep it warm for 1h, finish keeping it warm, and then cool down To 30°C, add 100g of ethyl acetate to raise the temperature and reflux, cool to 0°C, carry out suction filtration for crystallization, and dry the filter cake to obtain compound (4), 41.5g by weighing (93.8% yield, 99.0% chromatographic content).
Embodiment 3
[0044] Add 30.0g (0.1336mol) of 2,4,5-trifluoro-3-methoxy-benzoyl chloride (compound (2)) and 47.4g (0.1340mol) of tri-n-octylamine in the four-necked flask, After the addition, control the temperature at 21°C, add 19.3g (0.1350mol) of N,N-dimethylaminoethyl acrylate (compound 3) dropwise, after the dropwise addition, control the internal temperature at 72°C to keep the reaction, keep warm for 1h, finish keeping warm, then cool down To 30°C, add 100g of ethyl acetate to raise the temperature and reflux, cool to 0°C, carry out suction filtration for crystallization, and dry the filter cake to obtain compound (4), 42.1g by weighing (95.1% yield, 99.1% chromatographic content).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com