Metal-supported cell
A metal support and monomer technology, applied in solid electrolyte fuel cells, fuel cells, electrochemical generators, etc., can solve the problems of metal support deterioration, interface peeling between solid electrolyte layer and metal support, etc., and achieve interface peeling inhibition. , The effect of excellent adhesion and stable battery performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
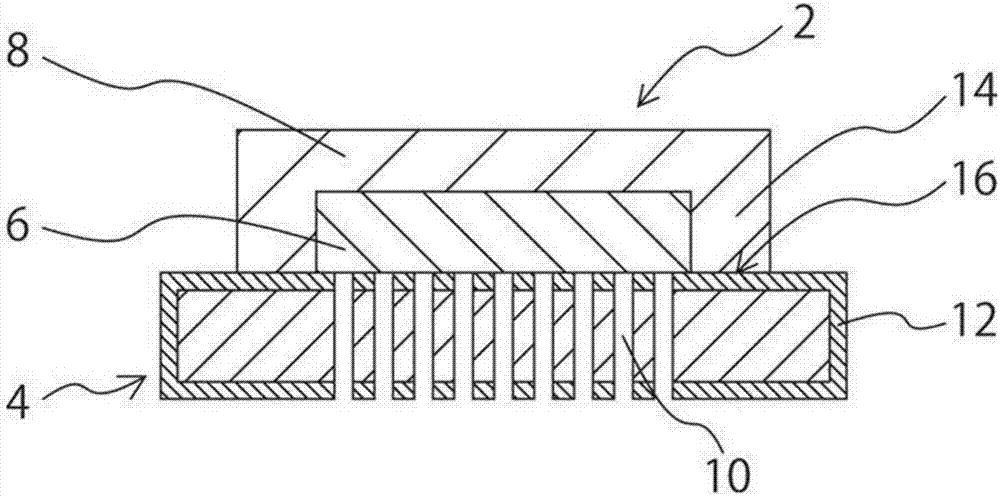
[0135] will have a diameter of The thickness of the support body is 250 μm, and in the center The locale has 140 cells / cm 2The ferritic stainless steel with through-holes was heat-treated at 600° C. for 1 hour to prepare a metal support having a chromium oxide layer with a film thickness of 1 μm on the surface.
[0136] Next, 5 parts by mass of ethyl cellulose, 5 parts by mass of ethyl cellulose, 40 parts by mass of α-terpineol were mixed to obtain a fuel electrode paste. The obtained fuel electrode paste was applied to the metal support by screen printing so as to cover the region having the through-holes of the metal support, dried at 120° C. for 1 hour, and then degreased at 450° C. for 2 hours. Then, the fuel electrode layer was formed on the metal support by firing at 800° C. for 2 hours under a nitrogen atmosphere. The obtained fuel electrode layer had a thickness of 18 μm and a ratio of NiO / (Ni+NiO) of 45 mol%.
[0137] Further, the solid electrolyte paste (1) pr...
Embodiment 2
[0139] After forming the fuel electrode layer on the metal support in the same manner as in Example 1, in Example 1, the solid electrolyte paste (1) was changed to the solid electrolyte paste (2) prepared in Preparation Example 2, except that Other than that, in the same manner as in Example 1, a solid electrolyte layer having a thickness of 18 μm was formed, thereby forming a semi-cell in which a fuel electrode layer and a solid electrolyte layer were sequentially stacked on a metal support. In the obtained semi-monomer, peeling from the fuel electrode layer and the metal support was not observed on the entire surface of the solid electrolyte layer, and the peeling evaluation was "3". The grain size of the solid electrolyte layer was 1.3 μm, the porosity was 2.5%, and the NiO / (Ni+NiO) ratio of the fuel electrode layer obtained after firing the solid electrolyte layer was 47 mol%. The width of the portion in direct contact with the metal support of the lower layer at the perip...
Embodiment 3
[0141] in diameter of The thickness of the support body is 250 μm, and in the center The locale has 140 cells / cm 2 On the surface of the through-hole ferritic stainless steel, a Ni-Co film was formed by plating treatment, and then heat-treated at 800°C for 1 hour, thereby preparing a Ni-Co-based tip with a film thickness of 5 μm on the surface. Metal support for the spar layer.
[0142] Next, 5 parts by mass of ethyl cellulose, α-terpene 40 parts by mass of pinol was mixed and homogenized to obtain a fuel electrode paste. The obtained fuel electrode paste was applied to the metal support by screen printing so as to cover the region having the through-holes of the metal support, dried at 120° C. for 1 hour, and then degreased at 450° C. for 2 hours. Then, firing was performed at 900° C. for 2 hours under a nitrogen atmosphere, whereby a fuel electrode layer was formed on the metal support. The obtained fuel electrode layer had a thickness of 20 μm and a ratio of NiO / (Ni+...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com