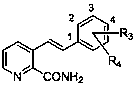
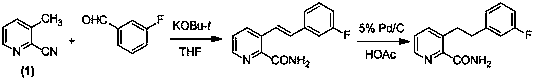
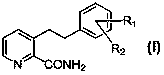
Preparation method of 3-(2-phenethyl)-2-pyridinecarboxamide compound
A picolinamide and compound technology, which is applied in the field of preparation of 3--2-pyridinecarboxamide compounds, can solve the problems of low yield, preparation limitation, and difficulty in obtaining reaction raw materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Preparation of 3-[2-(3-chlorophenyl)vinyl]-2-pyridinecarboxamide (3-17)
[0040] Add 2-cyano-3-picoline (10.0 mmol), 3-chlorobenzaldehyde (12.0 mmol) and N,N - Dimethylformamide (25 ml), after stirring evenly, add lithium hydroxide (3.0 mmol), stir the reaction at room temperature (the reaction progress is monitored by TLC), and the reaction is complete in about 24 h. After the reaction was completed, deionized water (50.0 ml) was added and extracted several times with ethyl acetate; the organic layers were combined and washed with deionized water and saturated NaCl aqueous solution; the organic layer was washed with anhydrous NaCl 2 SO 4 Dry, filter, evaporate the solvent under reduced pressure, and recrystallize the residue to obtain an off-white solid with a yield of 48.0%; mp 149~150 °C; 1 H NMR (CDCl 3 )d:8.48(d, J =4.4 Hz, 1H), 8.43(d, J =16.4 Hz, 1H), 8.04(d, J =8.0 Hz, 1H), 7.97(brs, 1H), 7.55(s, 1H), 7.46(dd, J =4.4 Hz, 8.0 Hz, 1H), 7.45(d, J =8.0 Hz,1...
Embodiment 2
[0042] Preparation of 3-[2-(3-chlorophenyl)vinyl]-2-pyridinecarboxamide (3-17)
[0043] The operation process was the same as in Example 1, except that lithium hydroxide was replaced by sodium hydroxide to obtain an off-white solid with a yield of 46.5%; its chemical structure was confirmed by NMR.
Embodiment 3
[0045] Preparation of 3-[2-(3-chlorophenyl)vinyl]-2-pyridinecarboxamide (3-17)
[0046] The operation process was the same as in Example 1, except that lithium hydroxide was replaced by potassium hydroxide to obtain an off-white solid with a yield of 54.0%; its chemical structure was confirmed by NMR.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com