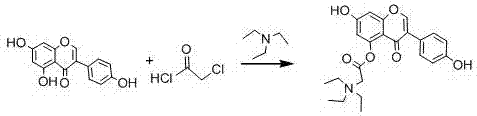
Acylated derivative of genistein and preparation process of acylated derivative
A technology of genistein and preparation process, which is applied in the field of pharmaceutical products, can solve the problems of no structural modification reports, etc., and achieve the effects of convenient mass production and application, simple operation, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
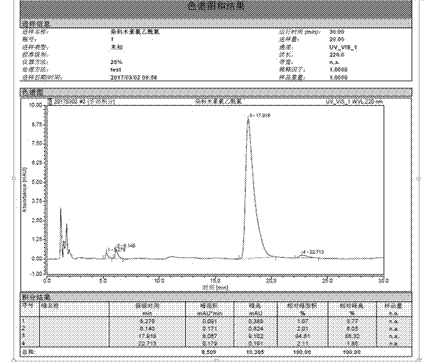
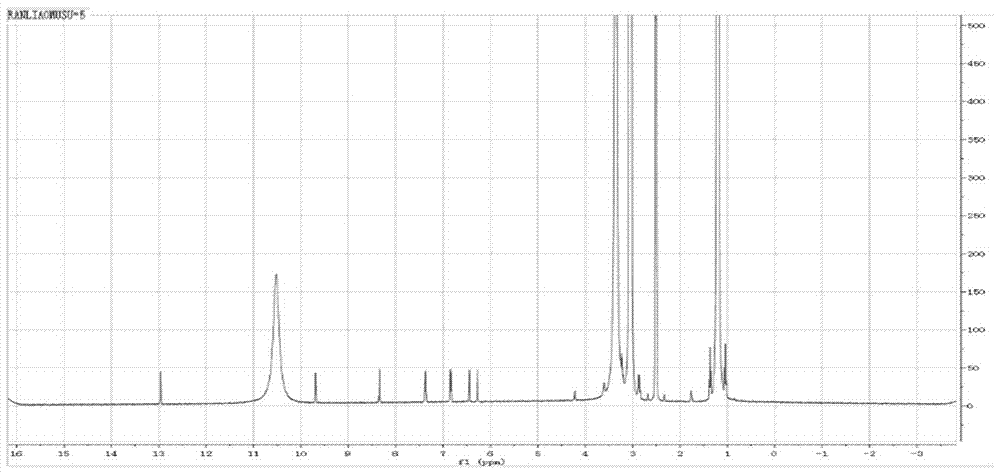
Image
Examples
Embodiment 1
[0031] Weigh 20g of genistein, put it into a 500ml three-neck round bottom flask, use 300ml tetrahydrofuran as a solvent, and add triethylamine dropwise to adjust the pH to about 5-7. Weigh 8.36 g of chloroacetyl chloride, dissolve it in 50 ml of tetrahydrofuran, and drop into the reaction solution at a rate of 6-10 drops per minute with a constant pressure dropping funnel. The reaction temperature was controlled at 0-5°C and monitored by thin-layer chromatography. After the reaction was completed, it was vacuum-filtered and vacuum-dried at 40°C.
Embodiment 2
[0033] Weigh 19g of genistein, put it into a 500ml three-neck round bottom flask, use 200ml of dichloromethane as solvent, and add triethylamine dropwise to adjust the pH to about 5-7. Weigh 9.36 g of chloroacetyl chloride, dissolve it in 45 ml of dichloromethane, and drop into the reaction solution at a rate of 6-10 drops per minute with a constant pressure dropping funnel. The reaction temperature was controlled at 0-5°C and monitored by thin-layer chromatography. After the reaction was completed, it was filtered with suction and dried under vacuum at 40°C.
Embodiment 3
[0035] Weigh 21g of genistein, put it into a 500ml three-neck round bottom flask, use 250ml dichloroethane as solvent, and add triethylamine dropwise to adjust the pH to about 5-7. Weigh 9.5 g of chloroacetyl chloride, dissolve in 40 ml of dichloroethane, and drop into the reaction solution at a rate of 6-10 drops per minute with a constant pressure dropping funnel. The reaction temperature was controlled at 0-5°C and monitored by thin-layer chromatography. After the reaction was completed, it was filtered with suction and dried under vacuum at 40°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com