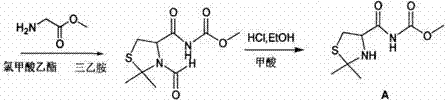
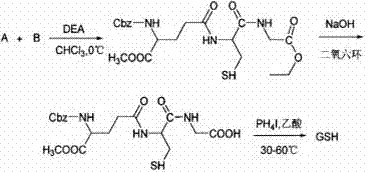
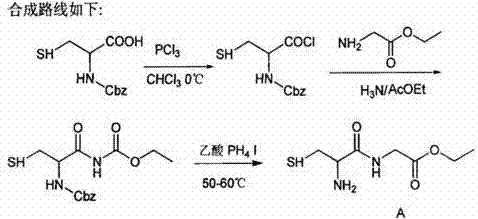
Compounding method for glutathione
A technology of glutathione and synthesis method, applied in the field of cosmetics and medicine, can solve the problems of unfriendly environment, high cost of chemical synthesis method, high toxicity of raw materials, etc., and achieve the effects of reducing cost, high purity and improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Preparation of Cystyl Chloride Phthalic Anhydride
[0050] In a 500mL reaction flask, add 120mL of toluene, 24g (0.1mol) of L-cystine, and 44.4g (0.3mol) of phthalic anhydride, then raise the temperature to 110°C, separate the water, and monitor the completion of the reaction by HPLC after the reaction solution is dissolved. Solids were precipitated, filtered, the filter cake was washed with 100ml of methanol, and dried to obtain 43.5g of cystine phthalic anhydride, with a yield of 86.9%.
[0051] Put 43.5g (0.086mol) of cystine phthalic anhydride into 150ml of chloroform, add 40.9g (0.344mol) of thionyl chloride dropwise, then raise the temperature to about 75°C and reflux for about 4 hours. After the reaction is dissolved, sample the derivative methyl ester for HPLC monitoring. After the reaction is complete, concentrate the net chloroform and excess thionyl chloride, and then add 150ml of chloroform to the oil for later use.
Embodiment 2
[0052] Embodiment 2: Preparation of glutathione
[0053] Add glycine 19.3g (0.258mol), toluene 20ml, HMDS 83.3g (0.516mol) into a 250mL reaction bottle, heat up to 110°C for reflux reaction for 2 hours, the reaction is complete after dissolution, it is glycine protected by HMDS, Cool down to 15°C for later use. Add the acid chloride in Example 1 dropwise into the HMDS-protected glycine, keep the temperature between 10-20°C to react, and react overnight after dropping, HPLC detects that the acid chloride has completely reacted, concentrated the solvent under reduced pressure, then added 200ml of water and stirred, and a large amount of solids Precipitated, filtered to obtain phtalic anhydride glutathione, and dried to obtain 43.9g with a purity of 94.7%.
[0054] In a 500ml reaction bottle, put 43.9g (0.068mol) of phtalic anhydride glutathione into 150ml of water, then add 7.6g (0.205mol) of 85% hydrazine hydrate, heat up to 70°C, react for 4h to sample HPLC raw materials, and...
Embodiment 3
[0055] Embodiment 3: tripeptide preparation
[0056] In a 1000mL reaction flask, add 64g (0.435mol) of glutamic acid, 300ml of toluene, and 96.5g (0.652mol) of phthalic anhydride, raise the temperature to about 110°C for reflux reaction, divide water, and monitor the reaction by HPLC after about 3 hours for the reaction solution to dissolve. After cooling down, solids were precipitated, filtered, the filter cake was washed with 120ml of methanol, and dried to obtain 98.8g of phthalic anhydride glutamic acid. In a 500ml reaction bottle, put 98.8g (0.357mol) of phthalic anhydride glutamic acid, then add 291.3g (2.85mol) of acetic anhydride, heat up to reflux, after 2 hours of reaction, TLC shows that the raw materials are completely reacted, cool down and filter, and use 100ml of ethyl acetate The ester was washed and dried to obtain 80.3 g (0.309 mol) of phthalic anhydride and glutamic anhydride.
[0057] In another 500ml reaction bottle, add 22.7g (0.064mol) of glutathione di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com