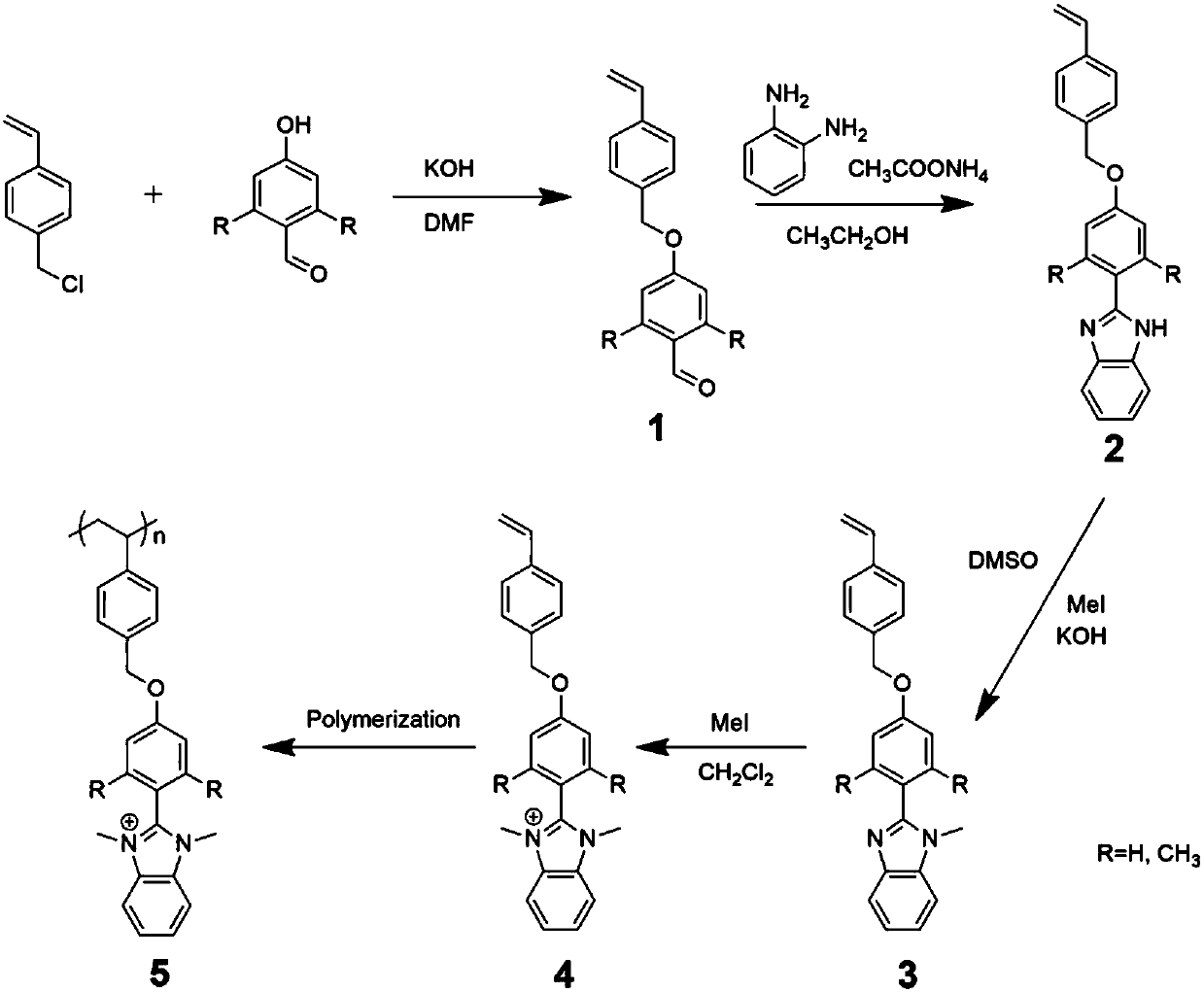
Novel benzimidazole ionic monomer and preparation method thereof
A technology of benzimidazole and ionic monomers, which is applied in the field of new benzimidazole ionic monomers and its preparation, and can solve the problems of inability to form ion clusters, limited applications, poor alkali resistance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Synthetic steps:
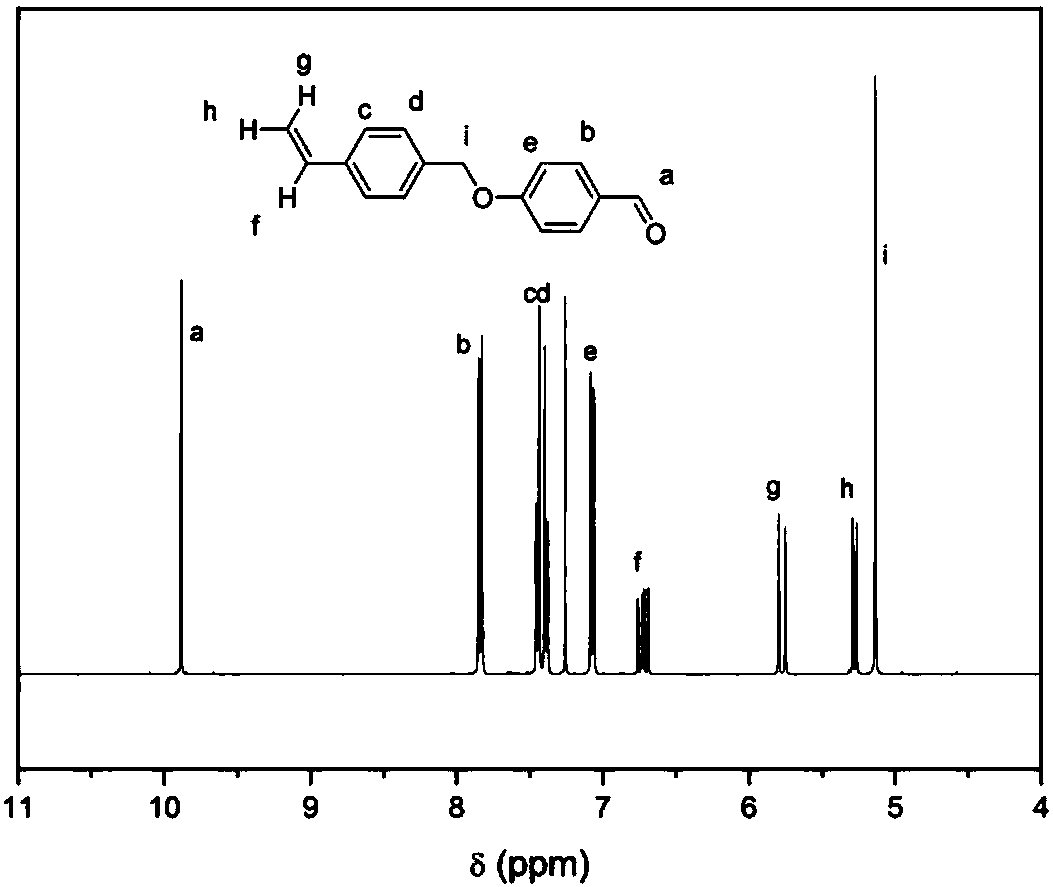
[0030] 1) 4-(4-vinylbenzyloxy)benzaldehyde (1a)
[0031] 6.7g (44.0mmol) 4-vinylbenzyl chloride, 4.89g (40.0mmol) 4-hydroxybenzaldehyde, 2.25g (40.0mmol) potassium hydroxide were dissolved in 80mL DMF, reacted at room temperature for 12h, poured into 800mL water, and precipitated White precipitate. Suction filtration, while washing with 200mL water to remove inorganic salts, and dry. Then, the unreacted raw material was removed by quenching with 200 mL of petroleum ether, suction filtered, and vacuum-dried to obtain 8.4 g of a white powder product (yield: 85%).
[0032] 2) 2-(4-(4-vinylbenzyloxy)phenyl)benzimidazole (2a)
[0033] 2.38g (10.0mmol) of 1a, 1.08g (10.0mmol) of o-phenylenediamine and 0.69g (10.0mmol) of ammonium acetate were dissolved in 150mL of absolute ethanol and refluxed at 80°C for 3h. After cooling, the solvent was removed in vacuo, and the obtained solid was separated by column chromatography to obtain 1.9 g of a yellow solid p...
Embodiment 2
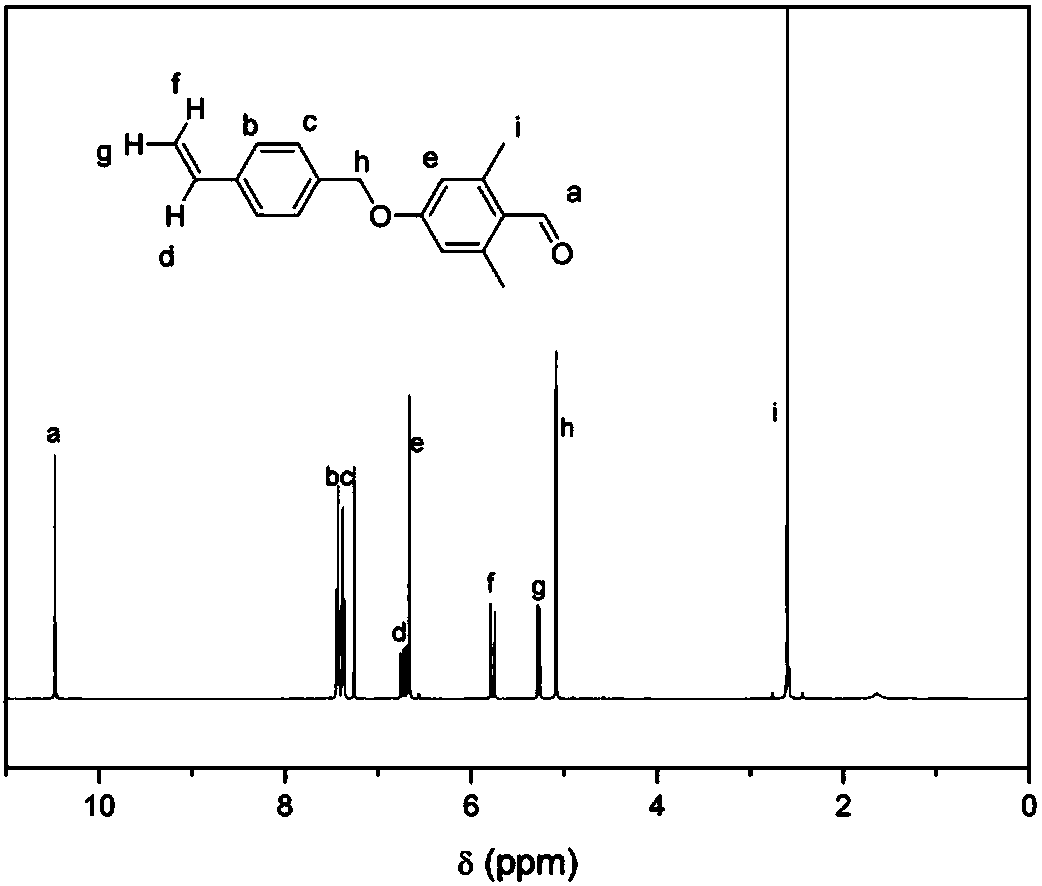
[0039] 1) 2,6-Dimethyl-4-(4-vinylbenzyloxy)benzaldehyde (1b)
[0040] 5.04g (33.0mmol) 4-vinylbenzyl chloride, 4.5g (30.0mmol) 2,6-dimethyl-4-hydroxybenzaldehyde, 1.68g (30.0mmol) potassium hydroxide were dissolved in 60mL DMF, and reacted at room temperature After 12 hours, it was poured into 800mL of water, and a beige precipitate was precipitated. Filter with suction while washing with 150 mL of water to remove inorganic salts, and dry. Then, the unreacted raw material was removed by quenching with 150 mL of petroleum ether, suction filtered, and vacuum-dried to obtain 6.1 g of beige crystal product (yield: 86%).
[0041] 2) 2-(2,6-Dimethyl-4-(4-vinylbenzyloxy)phenyl)benzimidazole (2b)
[0042] 2.66g (10.0mmol) of 1b, 1.08g (10.0mmol) of o-phenylenediamine and 0.69g (10.0mmol) of ammonium acetate were dissolved in 150mL of absolute ethanol and refluxed at 80°C for 3h. After cooling, the solvent was removed in vacuo, and the obtained solid was separated by column chromato...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com