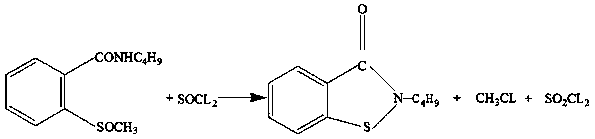
Synthetic method of N-n-butyl-1,2-benzisothiazolin-3-ketone
A technology of benzisothiazoline and synthesis method, applied in directions such as organic chemistry, can solve the problems of high price, high price of anhydrous lithium iodide, difficulty in generating target products and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
[0031] Add 0.30mol of about 50.4g of dry o-methylthiobenzoic acid and 41g of thionyl chloride to 200ml of dichloroethane, raise the temperature to 60°C, react for a period of time, and analyze the unreacted o-methylthiobenzene by gas chromatography HPLC Acyl chloride reaction is completed when formic acid ≤0.50%. The solvent dichloroethane was removed to obtain 53.7 g of o-methylthiobenzoyl chloride, with a yield of 96.0%. The HPLC analysis product contained 99.54% o-methylthiobenzoyl chloride and 0.46% o-methylthiobenzoic acid.
[0032] Add 0.30mol about 55.9g of o-methylthiobenzoyl chloride to 300ml of dichloroethane, add dropwise a mixture of 50.4g of n-butylamine and 50g of dichloroethane at room temperature, and raise the temperature to 80°C for 2 hours after adding , add 18.5g of soda ash, 50g of water, distill and recover redundant n-butylamine and dichloroethane, wash with water and filter to obtain 63.2g of solid N-n-butyl-o-methylthiobenzamide. The HPLC analysis pr...
Embodiment approach 2
[0036] Add 0.34mol about 58.1g of dry o-methylthiobenzoic acid and 52g of thionyl chloride to 200ml of dichloroethane, raise the temperature to 61°C, react for a period of time, and analyze the unreacted o-methylthiobenzene by gas chromatography HPLC Acyl chloride reaction is completed when formic acid ≤0.50%. After removing the solvent, 62.1 g of o-methylthiobenzoyl chloride was obtained, with a yield of 96.3%. HPLC analysis product contains o-methylthiobenzoyl chloride 99.60%, o-methylthiobenzoic acid 0.39%.
[0037] Add 0.31mol about 58.4g of o-methylthiobenzoyl chloride to 300ml of dichloroethane, add dropwise a mixture of 52.4g of n-butylamine and 52g of dichloroethane at room temperature, and heat up to 82°C for 2.5 h, add 23.1 g of soda ash and 60 g of water, distill and recover excess n-butylamine and dichloroethane, wash with water, and filter to obtain 66.3 g of solid N-butyl-o-methylthiobenzamide. The HPLC analysis product contained N-n-butyl o-methylthiobenzamide...
Embodiment approach 3
[0041] Add 0.36mol of about 61.2g of dry o-methylthiobenzoic acid and 48g of thionyl chloride to 200ml of dichloroethane, raise the temperature to 62°C, react for a period of time, and analyze the unreacted o-methylthiobenzene by gas chromatography HPLC Acyl chloride reaction is completed when formic acid ≤0.50%. After removing the solvent, 64.3 g of o-methylthiobenzoyl chloride was obtained, with a yield of 94.7%. The HPLC analysis product contained 99.44% o-methylthiobenzoyl chloride and 0.55% o-methylthiobenzoic acid.
[0042] Add 0.33mol about 61.5g of o-methylthiobenzoyl chloride to 300ml of dichloroethane, add dropwise a mixture of 55.0g of n-butylamine and 55g of dichloroethane at room temperature, and heat up to 81°C for 2.2 h, add 24.5 g of soda ash and 75 g of water, distill and recover excess n-butylamine and dichloroethane, wash with water and filter to obtain 69.1 g of solid N-butyl-o-methylthiobenzamide. According to HPLC analysis, the product contained 99.06% ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com