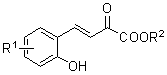
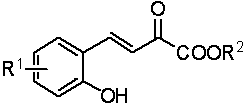
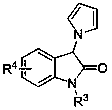
Chiral bridge ring skeleton oxindole piperidine compound and synthesis method of compound
A technology of oxindole spiropiperidine and its synthesis method, which is applied in the field of chiral nitroxide-bridged ring skeleton and spiro-ring oxindole compound and its synthesis, which can solve the problems of few reports on the synthesis method and achieve enantioselectivity And the effects of excellent diastereoselectivity, good yield, and good chemoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]
[0040] 1a (38.1 mg, 0.15 mmol, 1.5 equiv), 2a (21.2 mg, 0.1 mmol) and catalyst Cat. (4.1 mg, 0.01 mmol, 10 mol%) were placed in a test tube containing methyl tert-butyl ether (1.0 mL) , reacted at room temperature for 12 hours until the substrate 2a disappeared, and removed the solvent under reduced pressure. The resulting residue was separated by petroleum ether / ethyl acetate (2 / 1) column chromatography, and the obtained product was dissolved in toluene (1.0 ml) , and then added bistrifluoromethanesulfonimide (5.6 mg, 0.02mmol, 20 mol%) to the solution, and continued to react at room temperature for 12 hours, and the system was directly chromatographed with petroleum ether / ethyl acetate (3 / 1) Isolated to give 29.1 mg of 3aa as a white solid, 65% yield, 160–161 °C.
[0041] The product 3aa was analyzed and the results were as follows: >20:1 dr , 97% ee [Daicel Chiralcel AD-H, hexanes / i -PrOH = 80 / 20, flow rate: 1.0 mL·min –1 , λ = 254.4 nm, t (major)=5.857,...
Embodiment 2
[0044]
[0045] 1b (33.0 mg, 0.15 mmol, 1.5 equiv), 2a (21.2 mg, 0.1 mmol) and catalyst Cat. (4.1 mg, 0.01 mmol, 10 mol%) were placed in a test tube containing methyl tert-butyl ether (1.0 mL) , reacted at room temperature for 12 hours until the substrate 2a disappeared, and removed the solvent under reduced pressure. The resulting residue was separated by petroleum ether / ethyl acetate (2 / 1) column chromatography, and the obtained product was dissolved in toluene (1.0 ml) , and then add bistrifluoromethanesulfonimide (5.6 mg, 0.02mmol, 20 mol%) to the solution, continue the reaction at room temperature for 4 hours, and directly use petroleum ether / ethyl acetate (3 / 1) column chromatography Isolated to give 21.5 mg of 3ab as a white solid, 62% yield, 245–246 °C.
[0046] The product 3ab was analyzed and the results were as follows: >20:1 dr , 87% ee [Daicel Chiralcel AD-H, hexanes / i-PrOH = 80 / 20, flow rate: 1.0 mL min–1, λ = 254.4 nm, t (major) =9.448, t (minor) = 13.286...
Embodiment 3
[0049]
[0050] 1c (35.1 mg, 0.15 mmol, 1.5 equiv), 2a (21.2 mg, 0.1 mmol) and catalyst Cat. (4.1 mg, 0.01 mmol, 10 mol%) were placed in a test tube containing methyl tert-butyl ether (1.0 mL) , reacted at room temperature for 12 hours until the substrate 2a disappeared, and removed the solvent under reduced pressure. The resulting residue was separated by petroleum ether / ethyl acetate (2 / 1) column chromatography, and the obtained product was dissolved in toluene (1.0 ml) , and then add bistrifluoromethanesulfonimide (5.6 mg, 0.02mmol, 20 mol%) to the solution, continue to react at room temperature for 6 hours, and directly use petroleum ether / ethyl acetate (3 / 1) column chromatography Isolated to give 22.6 mg of 3ac as a white solid, 63% yield, 210–211 °C.
[0051] The product 3ac was analyzed and the results were as follows: >20:1 dr , 73% ee [Daicel Chiralcel AD-H, hexanes / i-PrOH = 80 / 20, flow rate: 1.0 mL min–1, λ = 254.4 nm, t (major) =10.739, t (minor) = 12.835]; [...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com