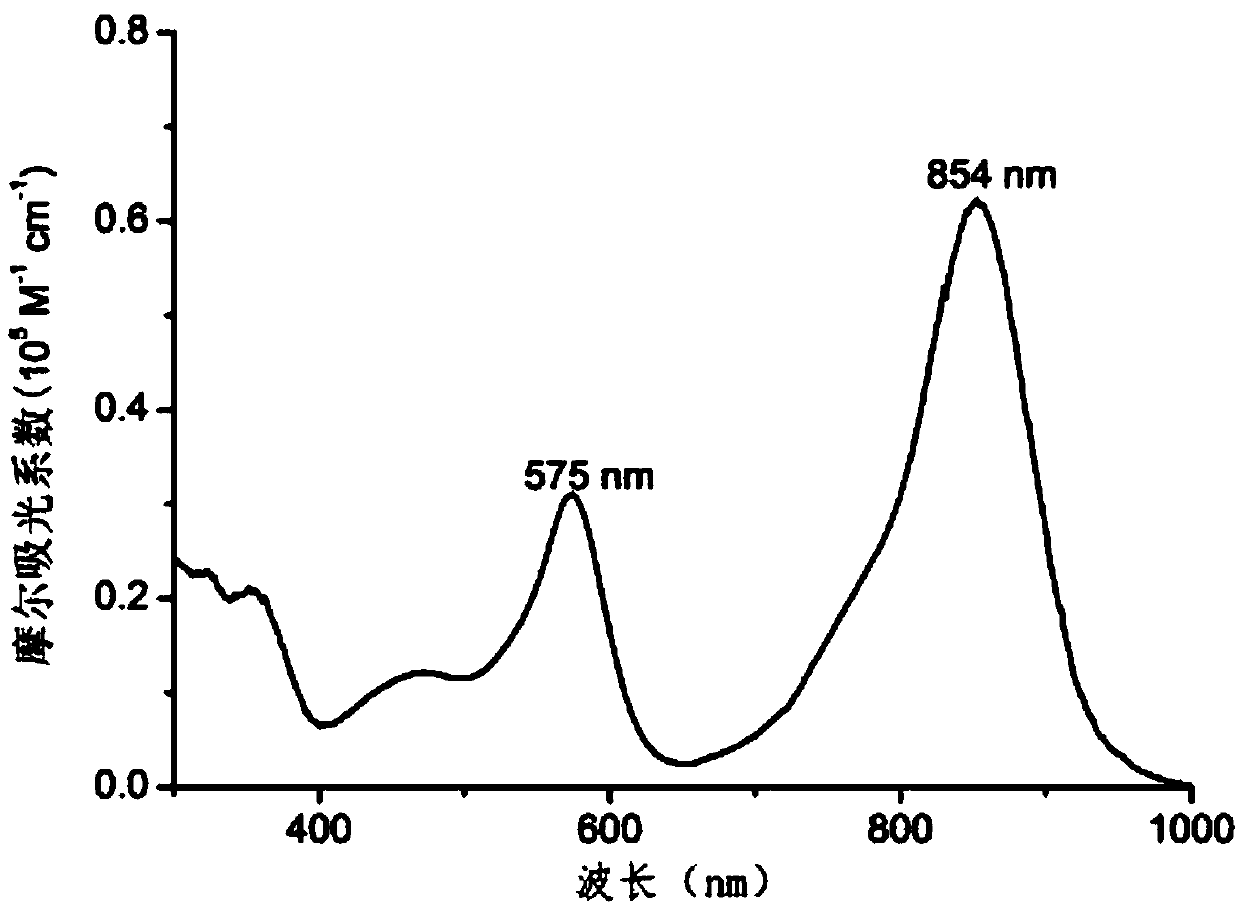
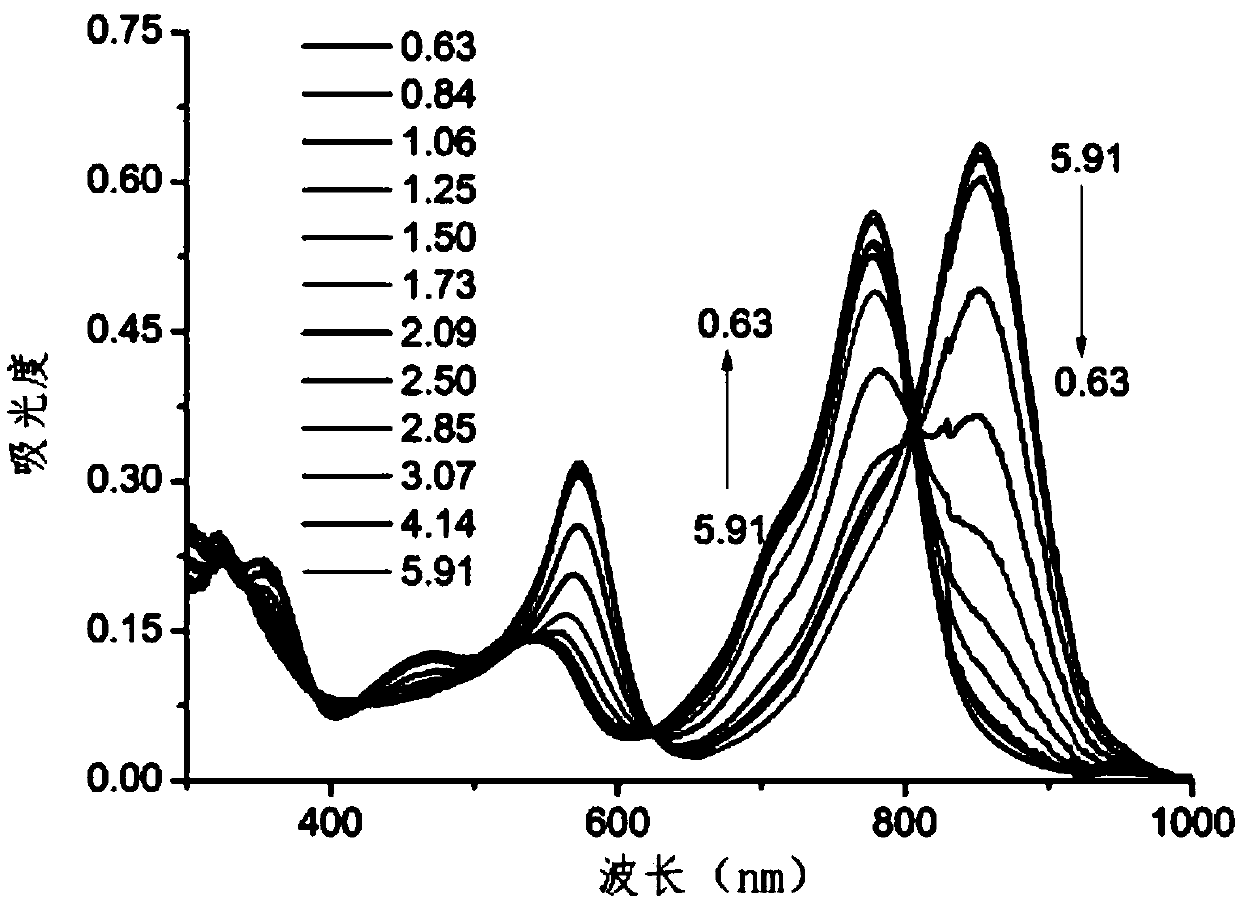
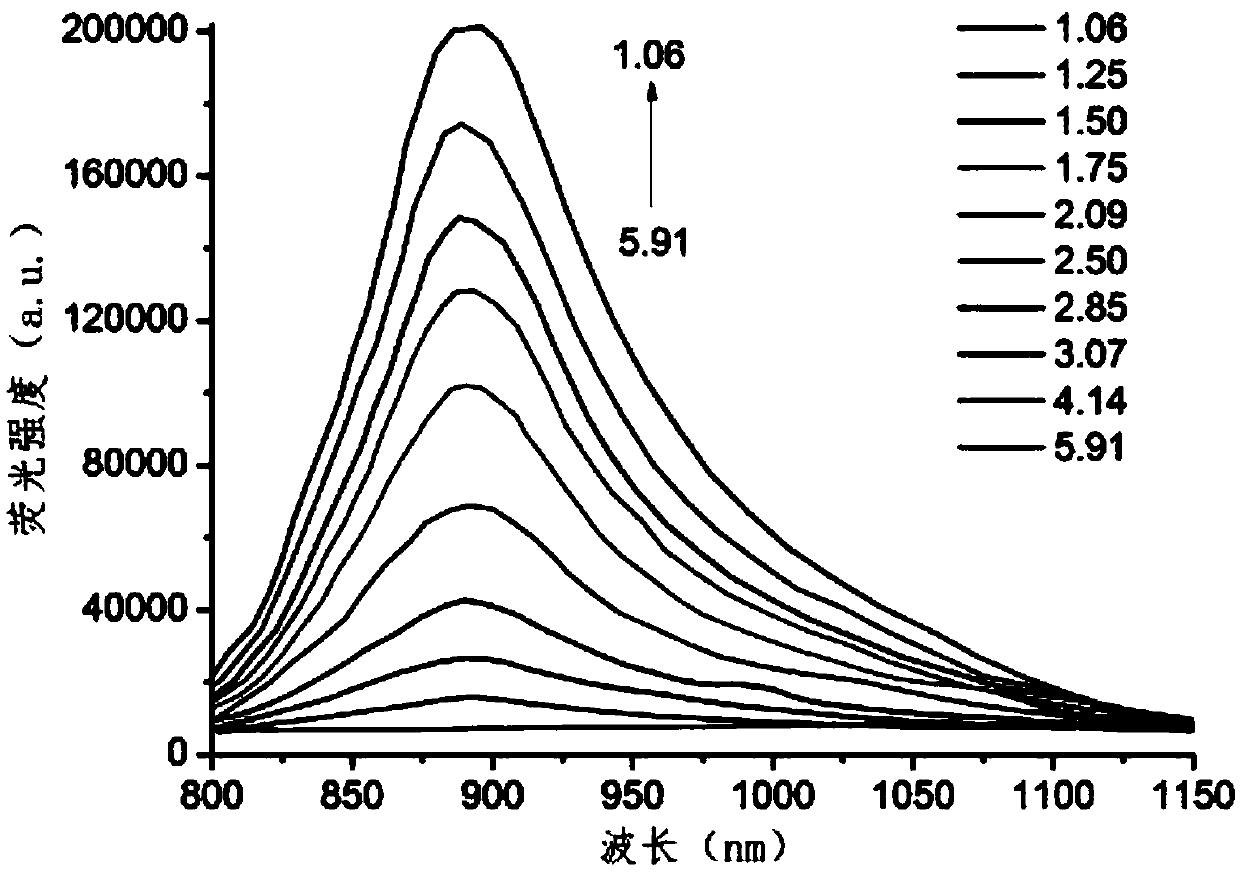
Amino-substituted BF2-chelated azadipyrromethene near-infrared pH fluorescent probe and preparation method and application thereof
A technology of heterofluoroboron dipyrrole and near-infrared light, which is applied in the field of near-infrared absorption pH fluorescent probe to achieve the effect of high selectivity and enhanced fluorescence emission
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1. Synthesis of compound 1a:
[0027] In a 100mL round bottom flask, add 10mmol 4-N, N-diethylamino-acetophenone and 10mmol benzaldehyde in 10mL ethanol solvent, slowly add (10mL) 10% NaOH aqueous solution dropwise, the reaction is over for 5 hours, and the pressure is reduced Suction filtration, washing with water until neutral, washing with a small amount of cold ethanol, and drying to obtain yellow product 1a.1a: 1 H NMR (500MHz, CDCl 3 , δ): 7.98 (d, J = 7.5Hz, 2H,), 7.70 (d, J = 13Hz, 2H,), 7.48-7.58 (m, 5H, Ar H), 6.68 (d, J = 5.5Hz, 2H, ), 3.44 (q, J=6Hz, 4H, CH 2 ), 1.22(t, J=6Hz, 6H, CH 3 ).ESI-MS (m / z): [M+Na] + calcd for C 19 h 21 NONa302.16; Found, 302.58.
Embodiment 2
[0028] The synthesis of embodiment 2. compound 2a:
[0029] Take (4mmol) 1a in a 50mL round bottom flask, 20mmol nitromethane and 20mmol diethylamine, 30mL methanol solvent, reflux at 60°C for 12 hours, after the reaction, the reaction mixture was extracted with distilled water and ethyl acetate, and then extracted with anhydrous sodium sulfate The organic layer was dried, the solvent was removed by rotary evaporation under reduced pressure, and the product 2a was obtained by drying. Zhou Jinfeng, Chu Chunjie; ID number of the first inventor: 41102319840416708
[0030] 2a: 1 H NMR (500MHz, CDCl 3 , δ): 7.79 (d, J=9.0Hz, 2H, Ar H), 7.44 (d, J=8.5Hz, 2H, Ar H), 7.17 (d, J=8.5Hz, 2H, Ar H), 6.58 (d, J=9.0Hz, 2H, Ar H), 4.81-4.85(m, 1H, CH), 4.62-4.66(m, 1H, CH), 4.10-4.21(m, 1H, CH), 3.42(q , J=7.0Hz, 4H, CH 2 ), 1.20(t, J=6Hz, 6H, CH 3 ).ESI-MS (m / z): [M+Na] + calcd for C 20 h 24 N 2 o 3 Na, 363.17; Found, 363.51.
Embodiment 3
[0031] Embodiment 3. Synthesis of compound 3a:
[0032] Take (3mmol) 2a in a 50mL two-neck round-bottom flask, add (75mmol) ammonium acetate, heat and reflux in 30mL n-butanol under nitrogen protection for 24h, cool to room temperature after the reaction is complete, filter, wash with cold ethanol, and dry to obtain dark blue Colored solid 3a.3a: 1 H NMR (500MHz, CDCl 3 , δ): 7.92 (d, J=8.5Hz, 4H, Ar H), 7.79 (d, J=8.5Hz, 4H, ArH), 7.52 (d, J=8.0Hz, 4H, Ar H), 7.07 ( d, J=8.0Hz, 4H, ArH), 6.78(d, J=8.5Hz, 4H, ArH), 3.48(q, J=7.0Hz, 8H, CH 2 ), 1.26(t, J=7.0Hz, 12H, CH 3 ).MALDI-TOF (m / z): calcd for C 40 h 41 N 5 , 591.79; Found, 591.71.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com