Organic electroluminescence material and organic luminescent device thereof
An electroluminescent material and organic technology, applied in the direction of luminescent materials, electric solid devices, electrical components, etc., can solve the problems of unsatisfactory luminous performance, high driving voltage, low luminous efficiency, etc., and achieve strong carrier transport ability, The effect of low driving voltage and high luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] The preparation method of a kind of organic electroluminescent material described in the present invention, comprises that shown raw material generates a kind of organic electroluminescent material shown in formula I by following route reaction:
[0053]
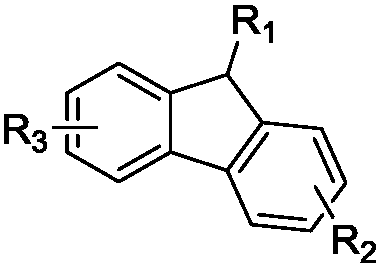
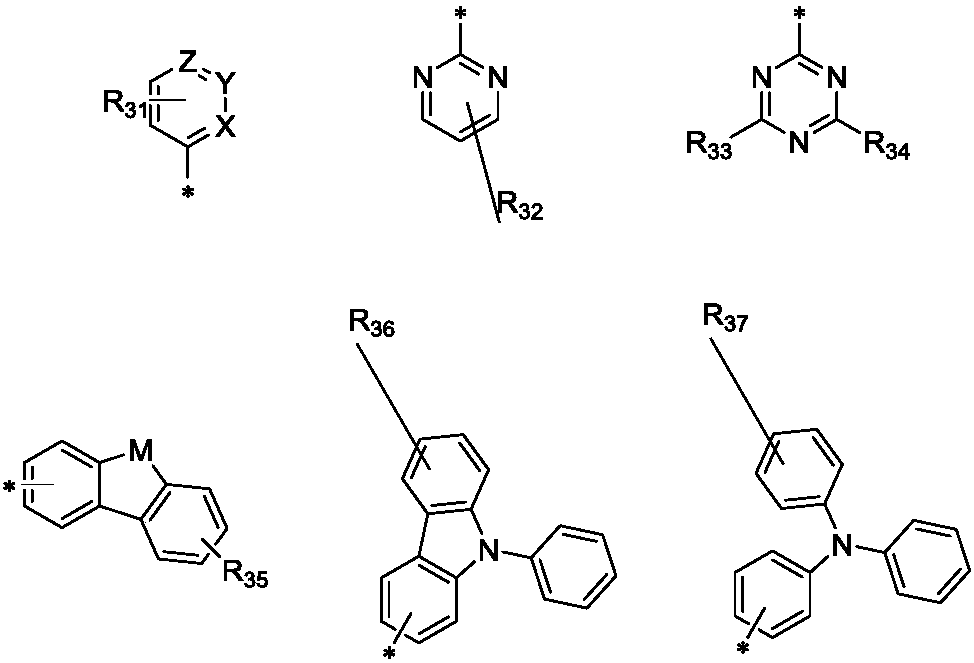
[0054] Among them, R 1One selected from substituted or unsubstituted C6-C50 aryl, substituted or unsubstituted C6-C50 arylamine, substituted or unsubstituted C6-C50 aryl ether, substituted or unsubstituted C3-C50 heteroaryl ;
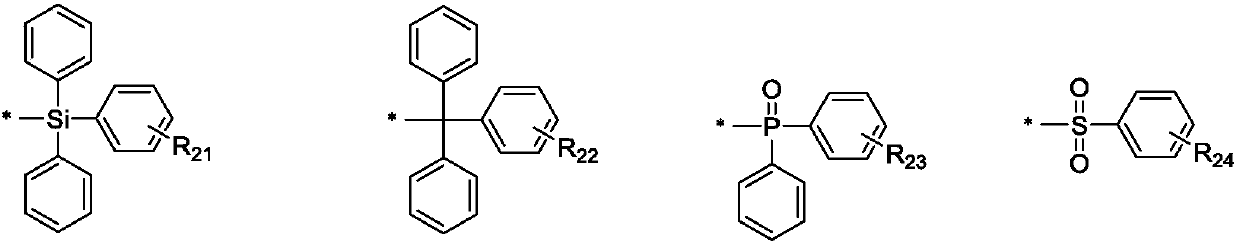
[0055] R 2 , R 3 independently selected from hydrogen, deuterium, cyano, substituted or unsubstituted C6-C50 sulfone group, substituted or unsubstituted C12-C50 phosphineoxy, substituted or unsubstituted C25-C50 tetraphenylmethyl, substituted Or one of the unsubstituted C18-C50 silaryl groups;
[0056] The heteroatom in the heteroaryl group is at least one of B, N, O, S, Si or P.
[0057] The present invention also provides an organic light emitting device, and the organic light emittin...
Embodiment 1
[0058] Embodiment 1: the preparation of compound 1
[0059]
[0060] Step1. Add 100 mmol of 3-bromofluorenone to a mixed solvent of solvent ethanol and toluene, add 3 equivalents of Raney nickel and 3 equivalents of hydrazine hydrate. Stir at room temperature for 5 hours, filter, concentrate the filtrate, and pass through column chromatography to obtain 75 mmol of product 1-1.
[0061] Step2. Dissolve 1-1 75mmol in anhydrous THF, cool down to -78 degrees Celsius, slowly add 150mmol of n-butyllithium dropwise, naturally warm up to room temperature, and react overnight. The next day, add 100mmol of triphenylchlorosilane and stir at room temperature 3h. Slowly warming up to reflux reaction for 3h. After the reaction was completed, it was passed through a silica gel column to obtain 1-2 55 mmol.
[0062] Step3. To 1-2 55mmol, add 60mmol of bromobenzene, 165mmol cesium carbonate, 0.6mmol palladium acetate, DMF, replace with argon three times, add 0.6mmol tricyclohexylphosphin...
Embodiment 2
[0063] Embodiment 2: the preparation of compound 2
[0064]
[0065] Step1. Add 100 mmol of 3,6-dibromo-9H-fluorenone to a mixed solvent of solvent ethanol and toluene, add 3 equivalents of Raney nickel and 3 equivalents of hydrazine hydrate. Stir at room temperature for 5 hours, filter, concentrate the filtrate, and pass through column chromatography to obtain 2-175 mmol of the product.
[0066] Step2. Dissolve 2-1 75mmol in anhydrous THF, cool down to -78 degrees Celsius, slowly add 225mmol of n-butyl lithium dropwise, naturally warm up to room temperature, and react overnight, the next day, add 200mmol of triphenylchlorosilane, room temperature Stir for 3h. Slowly warming up to reflux reaction for 3h. After the reaction was completed, it was passed through a silica gel column to obtain 2-2 55 mmol.
[0067] Step3. To 2-2 55mmol, add 60mmol of bromobenzene, 165mmol cesium carbonate, 0.6mmol palladium acetate, DMF, replace with argon three times, add 0.6mmol tricyclohex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com