A kind of tedizolid phosphate composition tablet
A technology of tedizolid phosphate and composition, which is applied in the field of tedizolid phosphate composition tablet, can solve problems such as unsatisfactory transportation and storage, unstable quality of finished products, inaccurate dosage forms, etc. Application, low cost, and improved antibacterial activity against MRSA
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
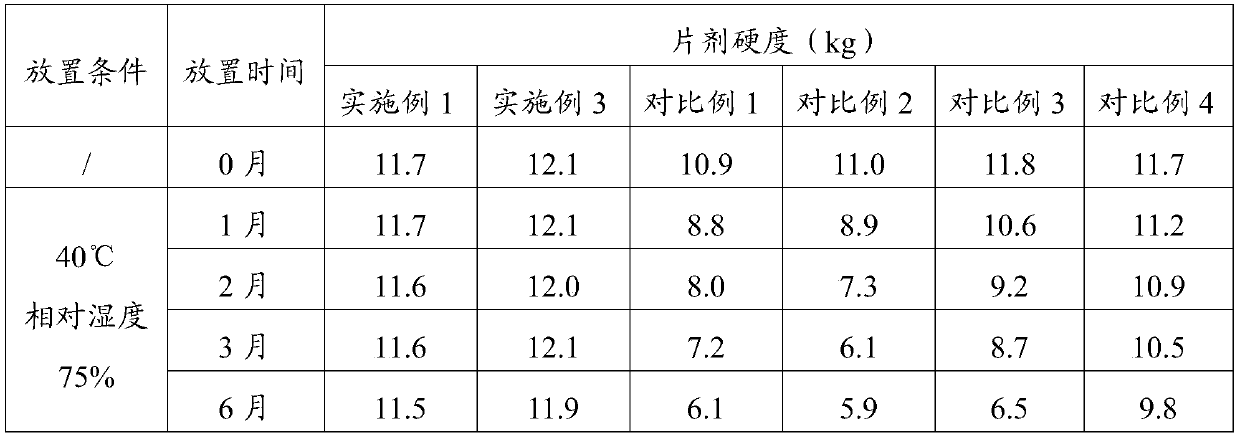
[0046] The investigation of experimental example 1 tablet hardness stability
[0047] Get the prepared tablet samples of Example 1, Example 3, Comparative Example 1, Comparative Example 2, Comparative Example 3, and Comparative Example 4, under packaging (aluminum-plastic packaging, put in a carton), at 40 ° C, Place it under 75% relative humidity for 6 months, take samples at 0, 1, 2, 3, and 6 months, and measure the tablet hardness. The results are shown in Table 1.
[0048] Table 1 tablet hardness investigation result
[0049]
[0050] The accelerated test was conducted for 6 months, and the hardness measurement results showed that the hardness of the tablet of the present invention remained unchanged under long-term storage conditions, and the stability of the preparation was significantly improved.
experiment example 2
[0051] Experimental example 2, dissolution test
[0052] Dissolution measurement method: the dissolution rate of the test product and the reference product was investigated according to the second appendix XD of the "Pharmacopoeia of the People's Republic of China" 2015 edition. 900ml of 0.05M phosphate buffer solution, pH 6.80±0.05 was used as the dissolution medium, the temperature was 37°C, the rotation speed was 50 rpm, and the amount of drug in each sample to be tested was 30mg. Samples were taken at 45 minutes by ultraviolet spectrophotometry for dissolution determination. The detection wavelength was 274nm, and the linear relationship was good within 5-25mg·L-1. The recovery rate and precision experiments all met the methodological requirements. The test results are shown in Table 2.
[0053] Table 2 Dissolution test results
[0054] Dissolution % Dissolution % Example 1 99.6 Comparative example 1 97.5 Example 2 99.5 Comparative example...
experiment example 3
[0055] Experimental Example 3: Efficacy and Safety Evaluation of Treatment for MRSA Pneumonia
[0056] Refer to the patent CN 105085570 A method for detection, and the test results are shown in Table 3-5.
[0057] Table 3 Comparison of Clinical Curative Effects of Patients (Example)
[0058] group Total effective rate, % group Total effective rate, % Example 1 88.15 Comparative example 1 82.05 Example 2 87.95 Comparative example 2 83.27 Example 3 87.99 Comparative example 3 86.23 Example 4 87.84 Comparative example 4 87.12 Example 5 88.07
[0059] Table 4 Comparison of Bacteriological Curative Effects of Patients (Example)
[0060] group Bacterial clearance rate, % group Bacterial clearance rate, % Example 1 95.16 Comparative example 1 78.43 Example 2 95.08 Comparative example 2 80.15 Example 3 95.11 Comparative example 3 91.45 Example 4 95.23 Comparative example...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com