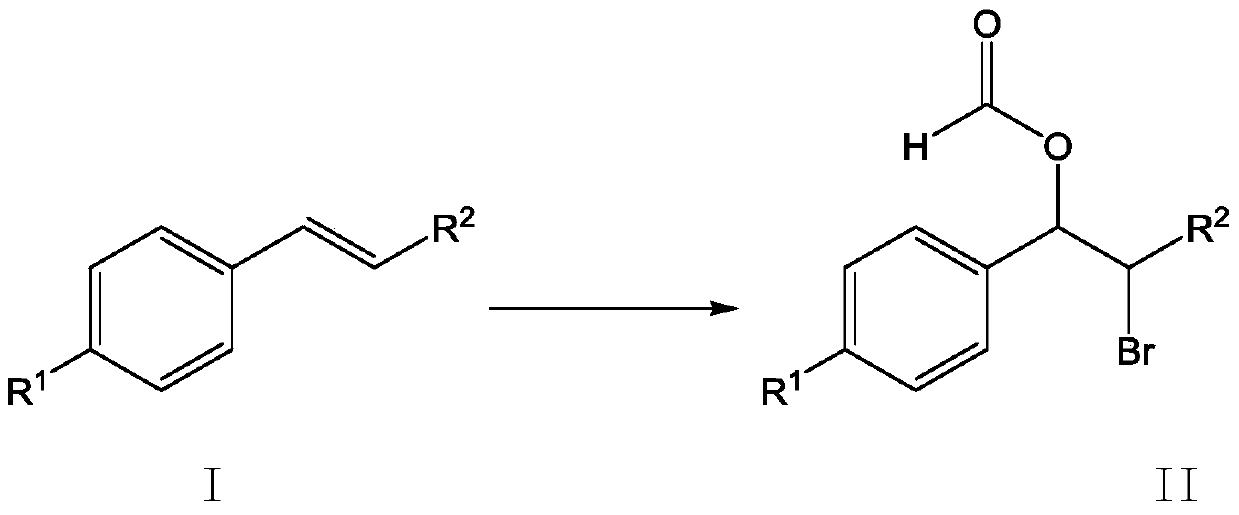
A method for synthesizing β-bromoformate compounds
A technology of ester compounds and compounds, which is applied in the field of synthesis of organic compounds, can solve the problems of environmental pollution costs, high toxicity, and poor stability of bromine sources, and achieve the effects of environmental friendliness, good stability, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]
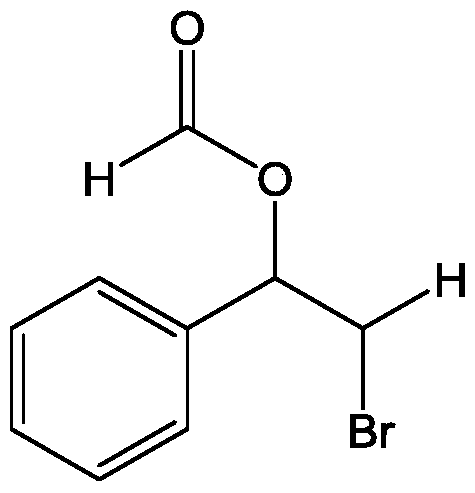
[0031] Add 2mmol (0.208g) of styrene and 1.8mmol (0.2142g) of potassium bromide into a 50mL three-necked flask, then add 10mL of formic acid as a solvent, and then add 0.8g of bromate-intercalated zinc-aluminum hydrotalcite ZnAl-BrO 3 - - LDHs, stirred magnetically at 40°C for 2 hours. After the reaction, the resulting reaction solution was centrifuged at 6500r / min to remove the zinc-aluminum hydrotalcite solid, the resulting liquid was placed in a separatory funnel, dichloromethane and deionized water were added, and the organic matter obtained from the reaction was extracted into the dichloromethane phase In the obtained solution, column chromatography silica gel was added, and the solvent was distilled off under reduced pressure, and the remaining mixture was separated by column chromatography, and the mixed solvent of petroleum ether and ethyl acetate with a volume ratio of 10:1 was used as the eluent to collect the product containing The eluent was evaporated...
Embodiment 2
[0034]
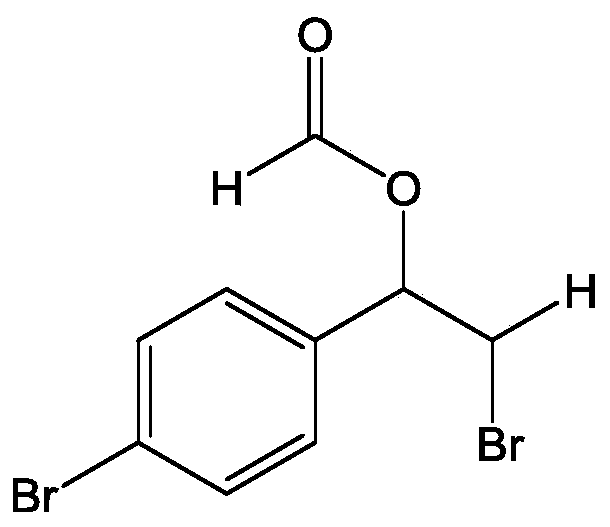
[0035] Add 2mmol (0.366g) of 4-bromostyrene and 1.8mmol (0.2142g) of potassium bromide into a 50mL three-neck flask, then add 10mL of formic acid as a solvent, and then add 0.8g of bromate-intercalated zinc-aluminum hydrotalcite ZnAl -BrO 3 - - LDHs, stirred magnetically at 50°C for 2 hours. After the reaction, the resulting reaction solution was centrifuged at 6500r / min to remove the zinc-aluminum hydrotalcite solid, the resulting liquid was placed in a separatory funnel, dichloromethane and deionized water were added, and the organic matter obtained from the reaction was extracted into the dichloromethane phase In the obtained solution, column chromatography silica gel was added, and the solvent was distilled off under reduced pressure, and the remaining mixture was separated by column chromatography, and the mixed solvent of petroleum ether and ethyl acetate with a volume ratio of 10:1 was used as the eluent to collect the product containing The eluent was evapo...
Embodiment 3
[0038]
[0039] Add 2mmol (0.277g) of 4-chlorostyrene and 1.8mmol (0.2142g) of potassium bromide into a 50mL three-necked flask, then add 10mL of formic acid as a solvent, and then add 0.8g of bromate-intercalated zinc-aluminum hydrotalcite ZnAl -BrO 3 - - LDHs, stirred magnetically at 50°C for 2 hours. After the reaction, the resulting reaction solution was centrifuged at 6500r / min to remove the zinc-aluminum hydrotalcite solid, the resulting liquid was placed in a separatory funnel, dichloromethane and deionized water were added, and the organic matter obtained from the reaction was extracted into the dichloromethane phase In the obtained solution, column chromatography silica gel was added, and the solvent was distilled off under reduced pressure, and the remaining mixture was separated by column chromatography, and the mixed solvent of petroleum ether and ethyl acetate with a volume ratio of 10:1 was used as the eluent to collect the product containing The eluent was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com