Preparation method of aripiprazole lauroxil
A technology of aripiprazole dodecanoate and alkyl, which is applied in the field of preparation of aripiprazole dodecanoate, can solve the problems of cumbersome operation, low yield, inconvenient industrial production, etc., and achieve reduction Production cost, the effect of facilitating industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0036] In order to make the object, technical solution and advantages of the present invention clearer, the present invention will be described in detail below in conjunction with the accompanying drawings and specific embodiments.
[0037] The meanings of the abbreviations used in the description of the present invention are: EA: ethyl acetate; THF: tetrahydrofuran; DMF: N,N-dimethylformamide; TLC: thin layer chromatography; TBAF: tetrabutylammonium fluoride.
[0038] The preparation method of aripiprazole laurate of the present invention comprises the steps:
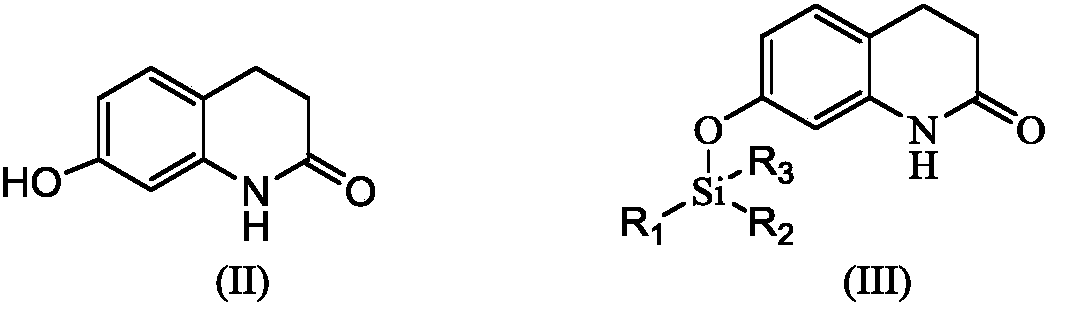
[0039] S1 In an amide solvent, compound (II) 7-hydroxyquinolinone and halosilane react to protect the phenolic hydroxyl group in the presence of an acid-binding agent to obtain compound (III),
[0040]
[0041] Wherein R1, R2, R3 are the same or different alkyl groups.
[0042] S2 In an alcohol solvent, compound (III) reacts with paraformaldehyde in the presence of an acid-binding agent, and the processed intermedi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com