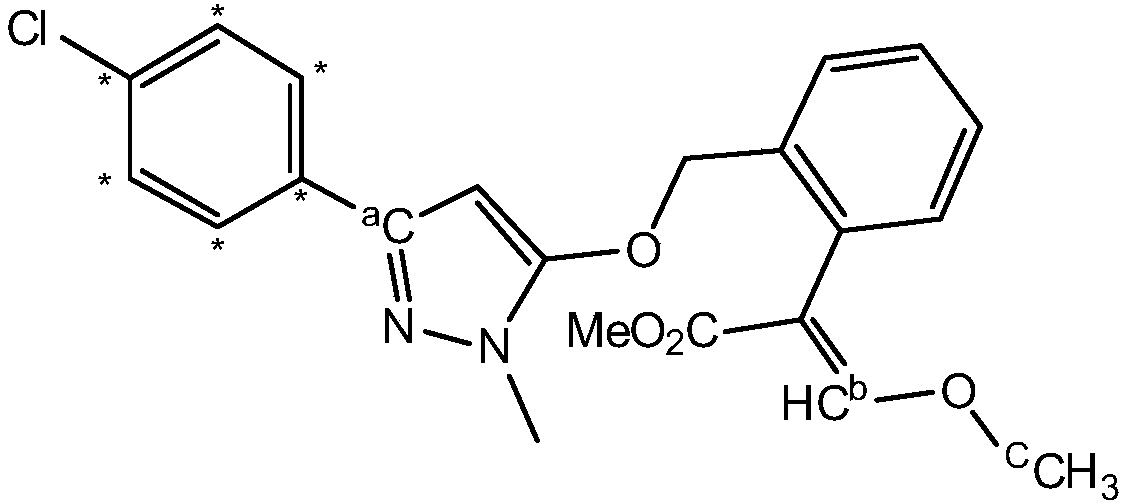
13C-labelled pyraoxystrobin and synthesis method thereof
A synthetic method, the technology of pyraclostrobin, applied in the field of stable isotope labeling synthesis, can solve the problems of many impurities, complex synthesis process, and difficult purification, and achieve the effect of easy separation and purification, simple operation process, and high atom utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
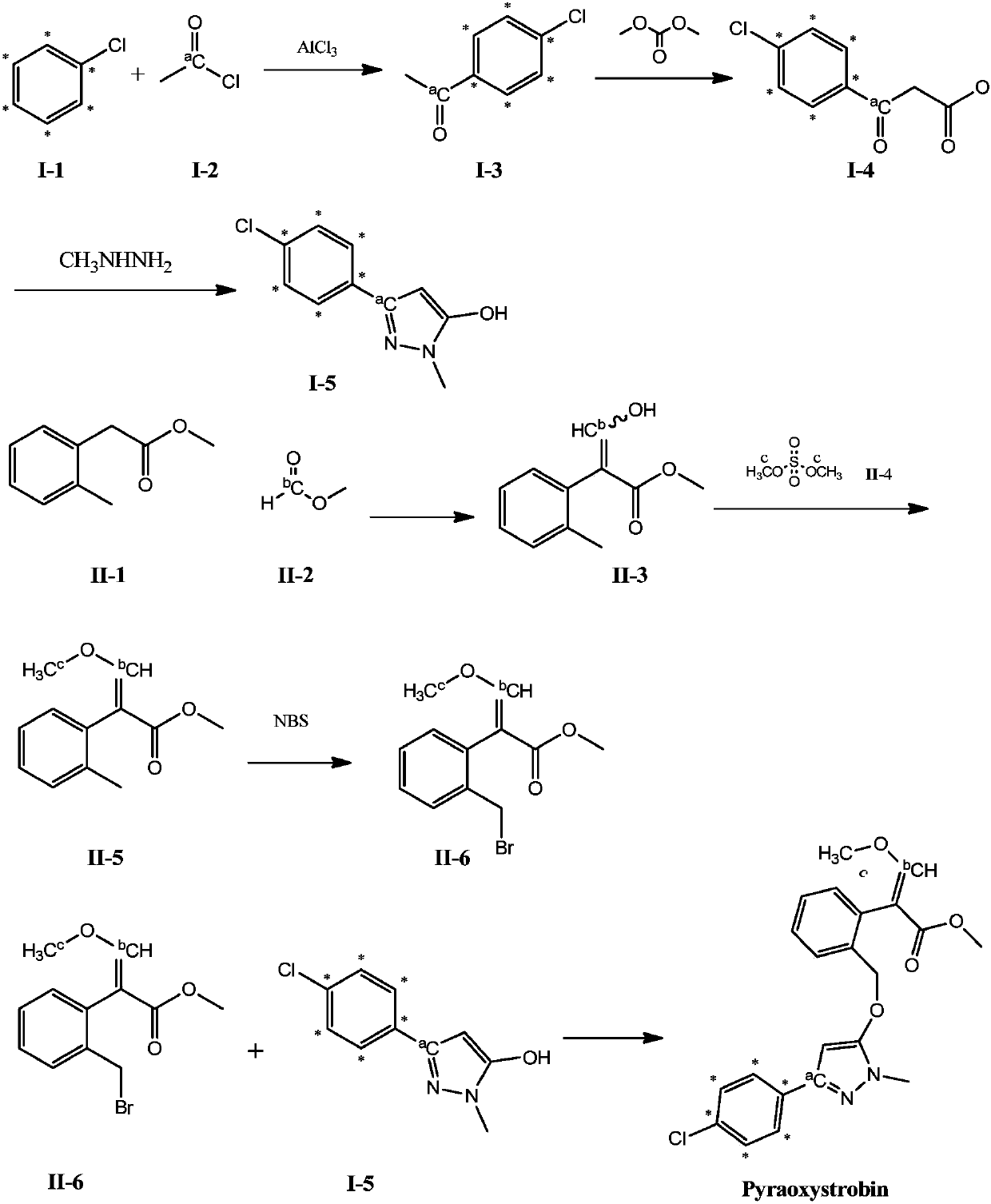
[0035] The present invention provides a 13 The synthetic method of the pyraclostrobin of C mark, comprises the steps:
[0036] Step (a): Using stable isotopes 13 The chlorobenzene of C mark is raw material, obtains the first product through acylation reaction, condensation ring reaction;
[0037] Step (b): Methyl o-toluene acetate undergoes condensation reaction, methylation reaction and bromination reaction to obtain the second product;
[0038]Step (c): the first product and the second product are reacted in an alkaline environment to obtain 13 C-labeled pyraclostrobin.
[0039] The synthesis method provided by the invention has simple operation process and high utilization rate of stable isotope atoms.
[0040] In a preferred embodiment, the acylation reaction in step (a) comprises acetylation reaction and formylation reaction;
[0041] The reagent of acetylation reaction is acetyl chloride; The reagent of formylation reaction is selected from dimethyl carbonate or met...
Embodiment 1
[0098] This embodiment provides a 13 C 7 A preparation method for marking pyraclostrobin, the method comprising the following steps:
[0099] (1) Preparation of 3-(4-chlorophenyl)-1-methyl-1H-5-pyrazolol (I-5)
[0100] Under nitrogen protection, aluminum trichloride (0.510g, 0.758mmol) and 13 C 6 Labeled chlorobenzene (0.7mL, 6.940mmol) was added to a 10mL stuffy jar, and acetyl chloride (0.500g, 6.289mmol) was added via a syringe at room temperature to seal it, and stirred at 55°C for 6h. After cooling, the reaction solution was quenched in ice water, extracted, and concentrated to obtain p-chloroacetophenone (0.890 g, yield 82.40%) as pale yellow oil. A three-necked flask protected by nitrogen was respectively charged with a tetrahydrofuran (10 mL) solution of p-chloroacetophenone (0.526 g, 3.372 mmol), and NaHMDS (2M in THF, 2.5 mL, 5.058 mmol) was added under an ice-salt bath and stirred for 0.5 h, and then Dimethyl carbonate (0.3 mL, 3.709 mmol) was added and the mix...
Embodiment 2
[0106] 13 C 7 A method for preparing pyraclostrobin, the method comprising the following steps:
[0107] (1) Preparation of 3-(4-chlorophenyl)-1-methyl-1H-5-pyrazolol (I-5)
[0108] Under nitrogen protection, aluminum trichloride (0.510g, 0.758mmol) and 13 C 6 Labeled chlorobenzene (0.7mL, 6.940mmol) was added to a 10mL stuffy jar, and acetyl chloride (0.500g, 6.289mmol) was added via a syringe at room temperature to seal it, and stirred at 55°C for 6h. After cooling, the reaction solution was quenched in ice water, extracted, and concentrated to obtain p-chloroacetophenone (0.890 g, yield 82.4%) as pale yellow oil. NaH (60%, 0.272g, 6.812mmol), tetrahydrofuran (10.0mL) and dimethyl carbonate (0.3mL, 3.709mmol) were respectively charged into a three-necked flask protected by nitrogen. The mixture was heated to reflux and stirred for 0.5 h, then a tetrahydrofuran solution (2.0 mL) of p-chloroacetophenone (0.526 g, 3.372 mmol) was added dropwise within 15 minutes, stirring ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com