Anti-GST tag protein nano antibody and application thereof
A technology for labeling proteins and nanobodies, applied in anti-enzyme immunoglobulins, applications, biochemical equipment and methods, etc., can solve the problems of short half-life and prolong half-life, and achieve the effect of good plasticity, high affinity and stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
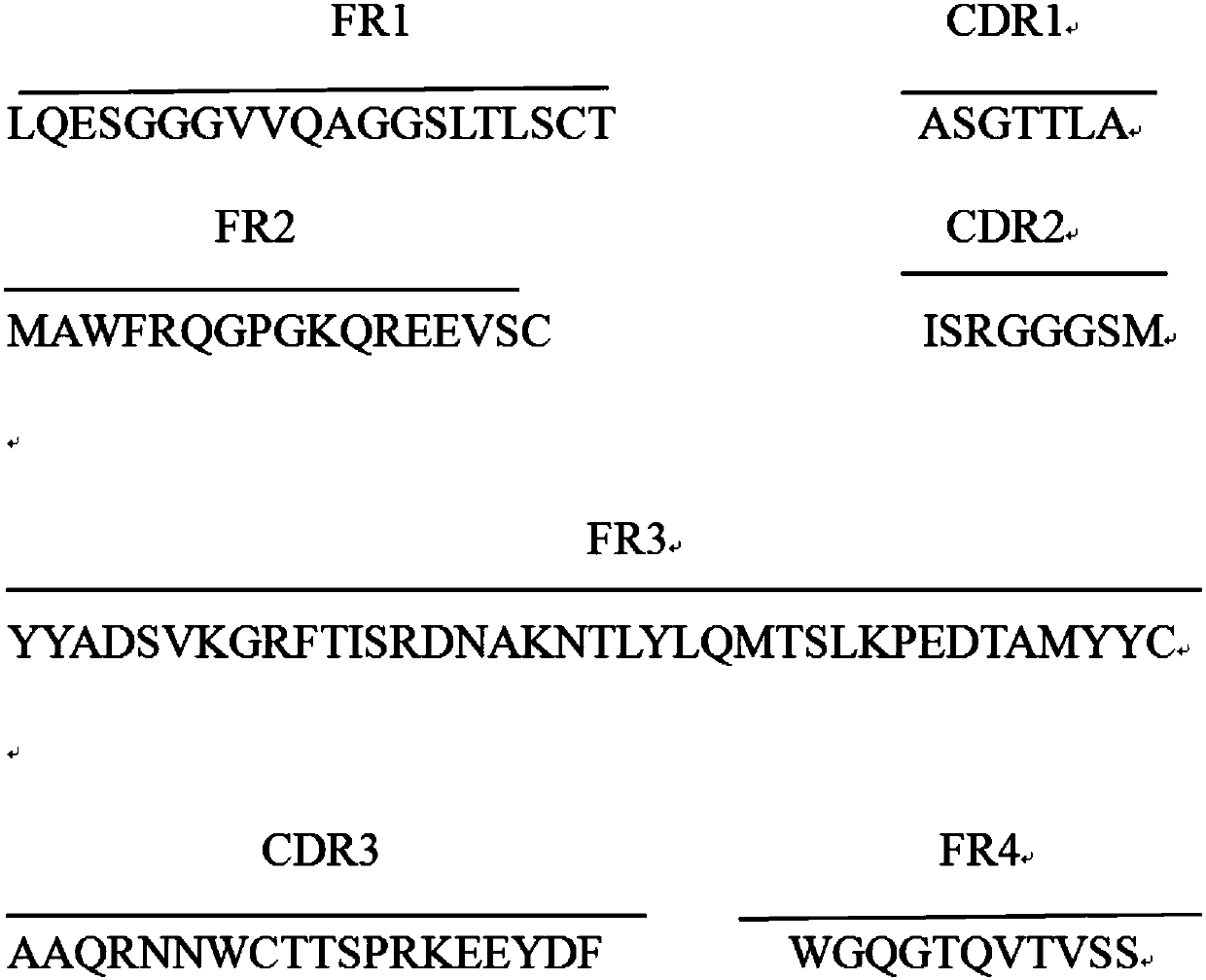
[0029] Example 1: Panning and Identification of Anti-GST Tag Protein Single Domain Heavy Chain Antibody
[0030] Using the method of solid-phase panning, the GST-tagged protein was diluted to 30-100ug / ul with 1xPBS, coated on the ELISA plate, and 100ul was added to each blank, and coated overnight at 4°C. Wash three times with PBS, add 300ul 4% skimmed milk to each well, and block for 2 hours at 37°C. After washing three times with PBS, add the phage display library (approximately 1x10 12 CFU), incubated at 37°C for 1 hour. Aspirate unbound phage, add PBS to wash 5-10 times (increase the number of washes with each round), and then wash three times with PBST, add 100ul of glycine-hydrochloric acid solution with pH=2.2, and incubate at 37°C for 5 minutes. Gently blow the wells of the plate to wash off the adsorbed phages, then add 15ul of Tris-Hcl solution (PH=8.8), take 10ul to measure the titer, and the rest will be amplified and used for the next round of panning.
[0031]...
Embodiment 2
[0044] Example 2: Expression and purification of Nanobodies
[0045] The above-mentioned base sequence was sent to the biological company and inserted into the PET28a plasmid to construct an expression vector. Take 1 ul of the constructed PET28a plasmid and add it to 50 ul of BL21 competent, place it on ice for 30 minutes, then place it in a 42° water bath for heat shock for 90 seconds, place it on ice for 5 minutes, then add 600 ul of LB medium and put it in 37 ° Incubate for 1 hour in an incubator. Take 100ul of the above-mentioned culture solution and spread it on the ampicillin plate, put it into a 37° constant temperature incubator and cultivate it overnight. The next day, pick a single clone from the plate and inoculate it into 1L culture medium, culture at 37°, 220rmp / min, when the bacterial solution OD=0.5, add IPTG with a final concentration of 0.1mM, 16°, 180rmp / min, overnight induced. The next day, the bacterial liquid was collected by centrifugation and resuspen...
Embodiment 3
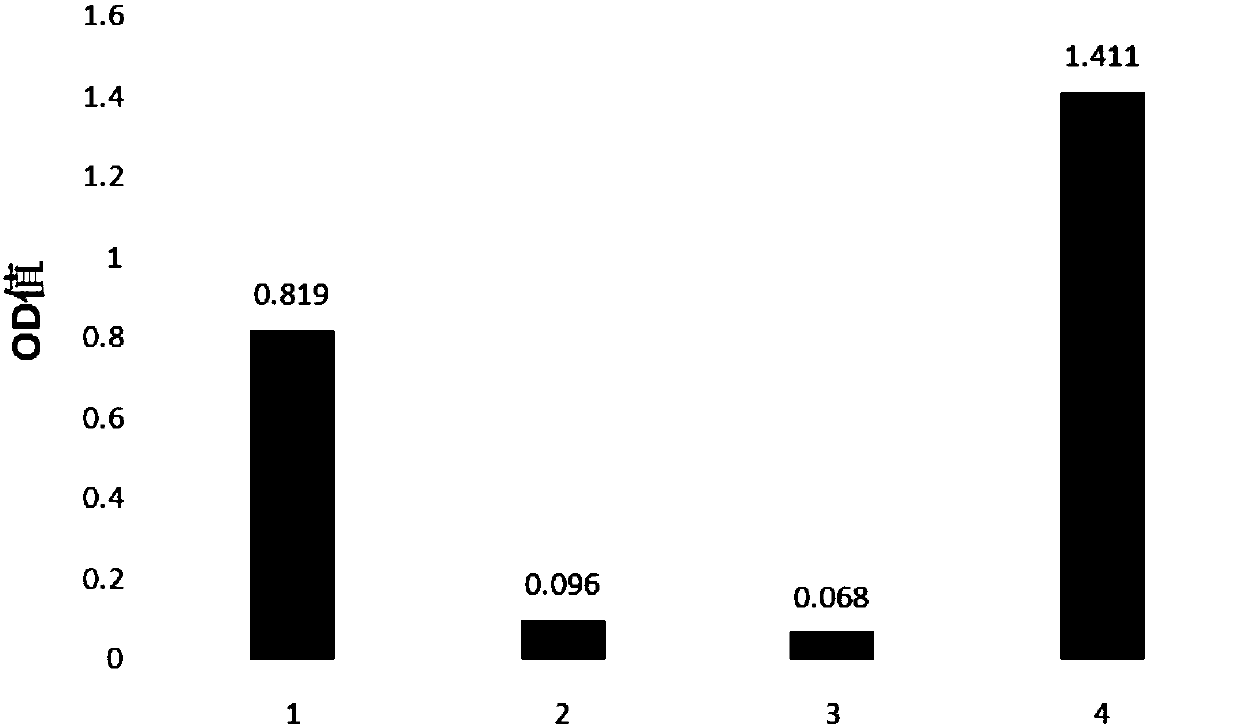
[0046] Example 3: Western blot verification
[0047] Western blot step: Take 500ng of anti-GST tagged protein, load the sample, electrophoresis with sodium dodecylsulfonate-polyacrylamide gel (SDS-PAGE), transfer to membrane, add specific primary antibody and corresponding secondary antibody successively for incubation , and then analyzed the effect of anti-GST tag protein nanobody by chemiluminescence colorimetry.
[0048] 1. Protein denaturation
[0049] Mix the protein sample whose concentration has been determined with 5× loading buffer solution at a ratio of 4:1, cook in boiling water for 10 minutes to denature the protein, and store it at -70°C after aliquoting for later use or directly use it for loading.
[0050] 2. Preparation of separating gel
[0051] 1) Prepare two matching 1.5mm glass plates, including a thin plate and a thick plate;
[0052] 2) Rinse the glass plate with tap water and dry it;
[0053] 3) Wash the comb with water and let it dry naturally;
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com