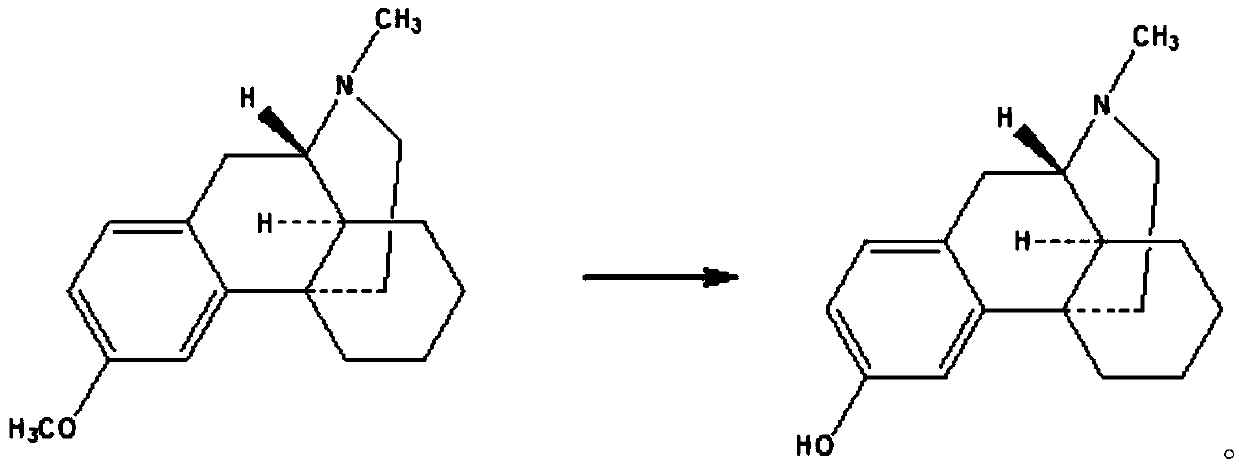
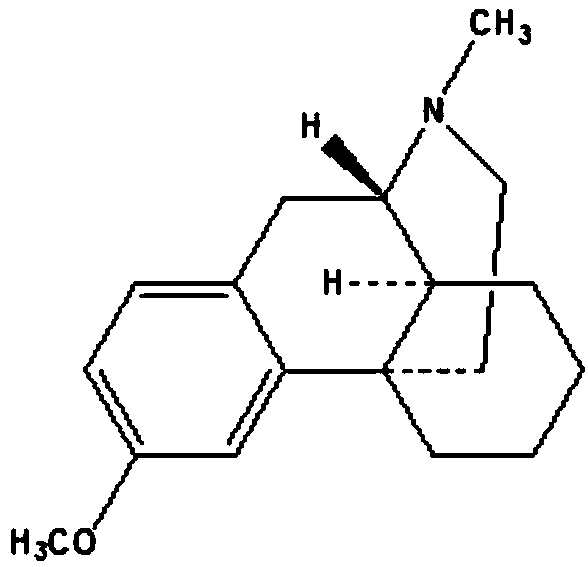
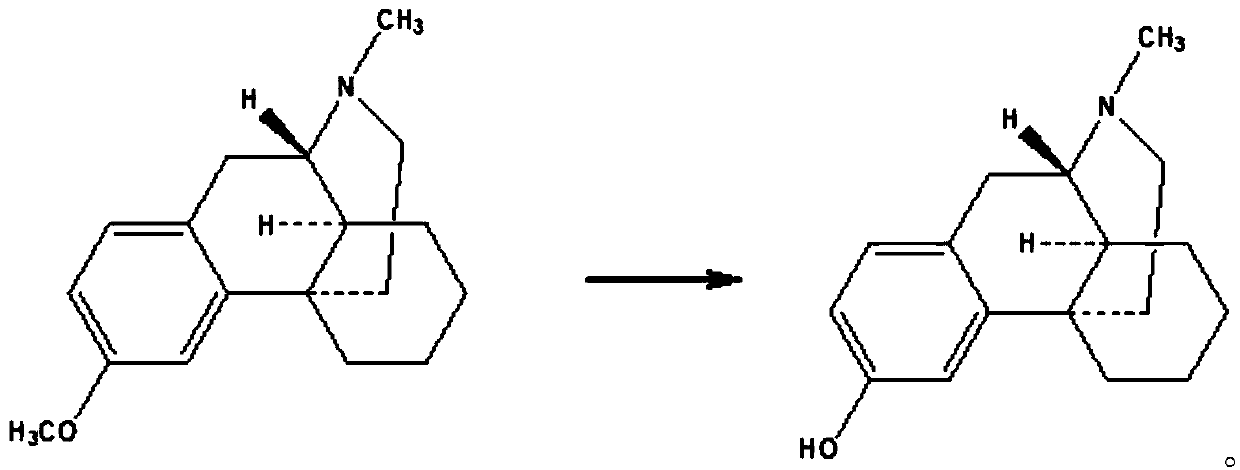
Preparing method of dextromethorphan impurity 17-methylmorphinan-3-alcohol enantiomer (DXM-B)
A technology of methylmorphinan and enantiomer, which is applied in the field of preparation of dextromethorphan impurity 17-methylmorphinan-3-ol enantiomer, can solve the problem of DXM-B preparation which has not been reported in literature Methods and other issues, to achieve the effect of cheap and easy-to-obtain raw materials, consistent purity, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
[0017] Example 1 Synthesis of 17-methylmorphinan-3-ol enantiomer (DXM-B)
[0018]
[0019] Put dextromethorphan hydrobromide monohydrate (20g, 54.05mmol), 48% hydrobromic acid (50g, 296.3mmol) and water (20g) into the reaction flask, heat up to reflux, and react for 24 hours. Cool down to room temperature, add water (100g), toluene (50g) and stir at room temperature. Adjust the pH to 8-9 with caustic soda (about 10 g), and stir for 15 minutes. The layers were allowed to stand, and the water layer was discarded. Water (100g) and caustic soda (4g, 100mmol) were added to the toluene layer and stirred for 15 minutes, the layers were left to stand, and the toluene layer was discarded. The aqueous layer was acidified with hydrochloric acid to pH 7-8, and a white solid was precipitated. Filtration, filter cake was added in 50% acetone water (50g), heated to dissolve, cooled to room temperature, precipitated white crystals, filtered, vacuum-dried to obtain 8.2 grams of product, ...
Embodiment 2
[0021] Put dextromethorphan base (20g, 73.8mmol), acetonitrile (120ml), boron trifluoride ether (25g, 176.1mmol) into the reaction bottle, under the protection of nitrogen, react at room temperature for 6 hours. The reaction solution was slowly added to water (200 g) and toluene (100 g), adjusted to pH 8-9 with liquid caustic soda, and stirred at room temperature for 15 minutes. The layers were allowed to stand, and the water layer was discarded. Water (100g) and caustic soda (6g, 150mmol) were added to the toluene layer and stirred for 15 minutes, the layers were left to stand, and the toluene layer was discarded. The aqueous layer was acidified with hydrochloric acid to pH 7-8, and a white solid was precipitated. Filtration, filter cake was added in 50% acetone water (80g), heated to dissolve, cooled to room temperature, precipitated white crystals, filtered, vacuum-dried to obtain 11 grams of product, yield 58%, purity 99.0% (HPLC, area normalized Chemical calculation)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com