Eszopiclone oral disintegrating tablet and preparation method thereof
A technology for eszopiclone and orally disintegrating tablets is applied in the directions of pharmaceutical formulations, medical preparations without active ingredients, medical preparations containing active ingredients, etc. Problems such as slow dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~9
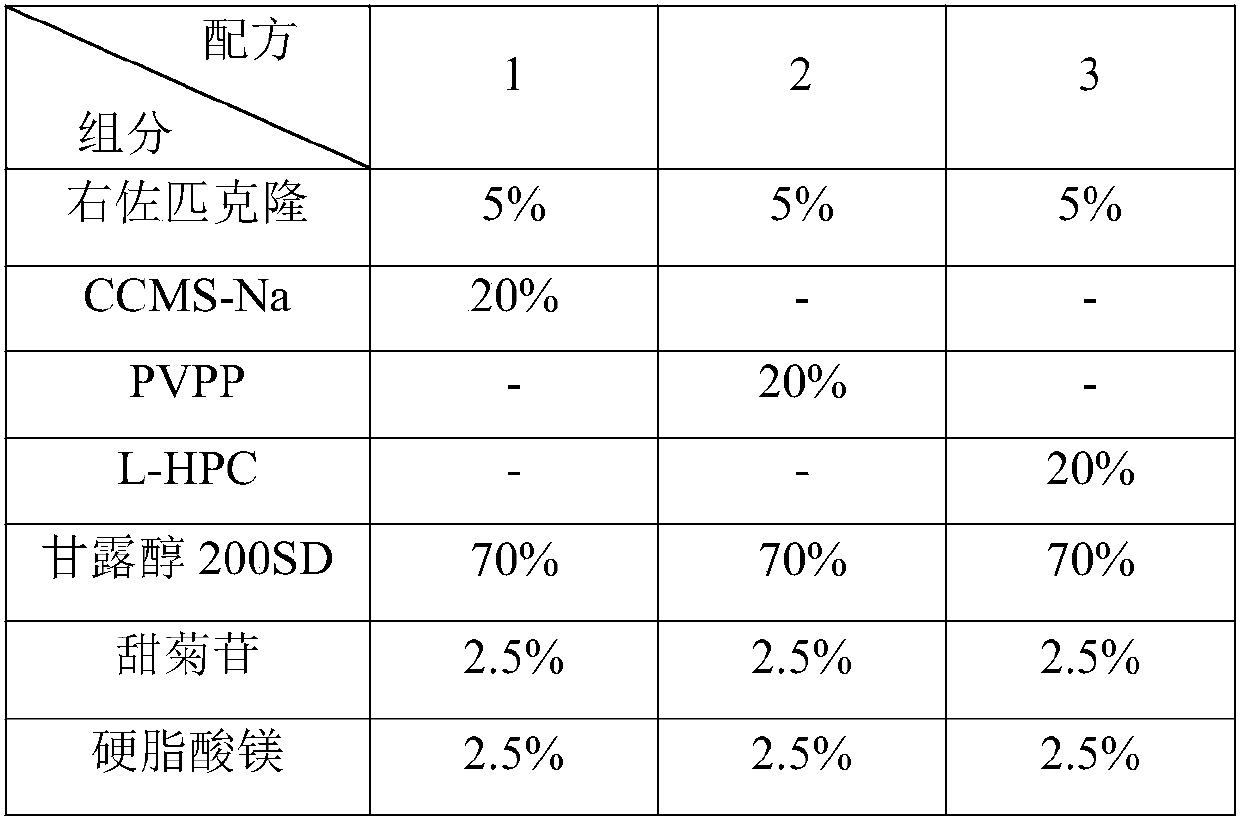
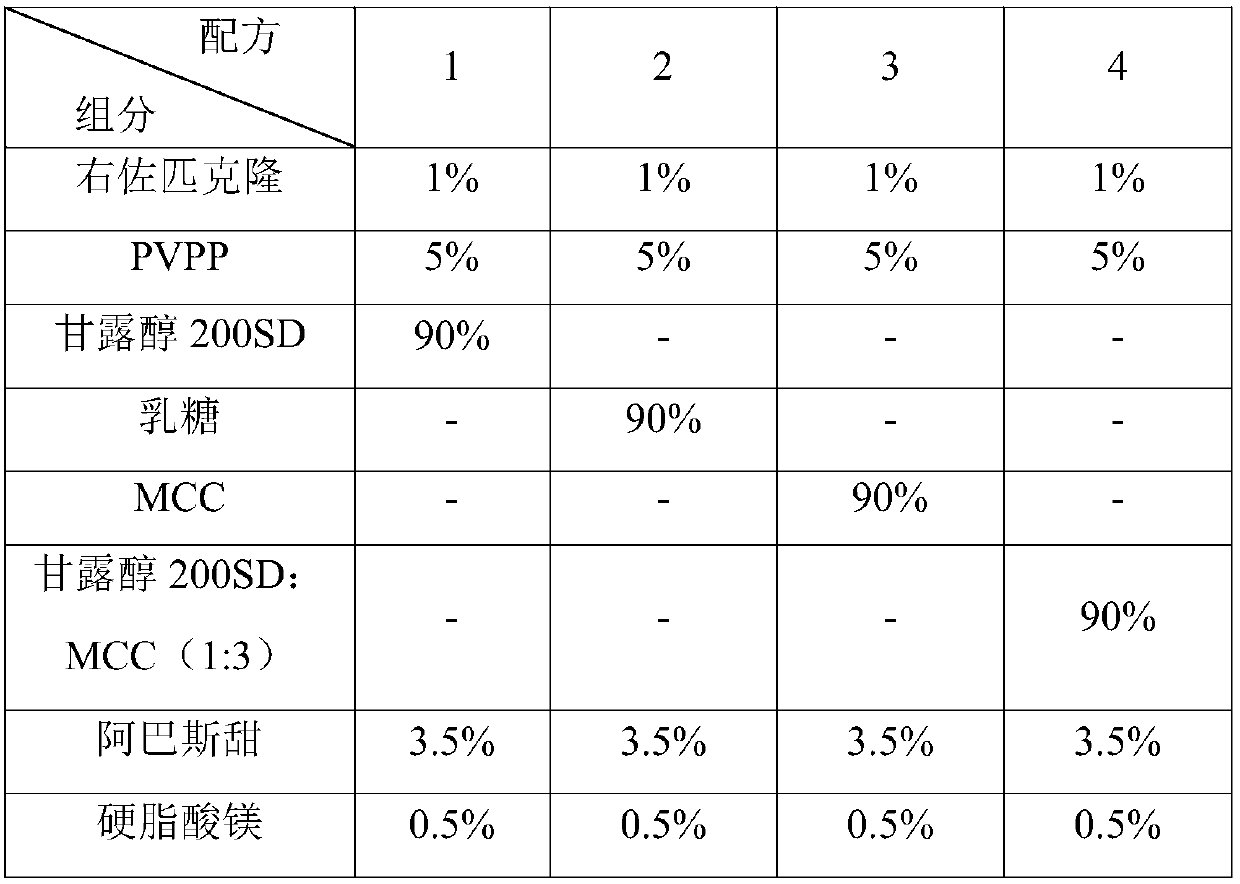
[0085] The orally disintegrating tablet of eszopiclone provided by the present invention is prepared from raw materials and auxiliary materials, wherein the raw material is eszopiclone, and the auxiliary materials include PVPP, a mixture of mannitol 200SD and MCC, stevioside, magnesium stearate, manna The weight ratio of alcohol 200SD to MCC is 1:4, by weight percentage, the ratio of each component is shown in Table 7.
[0086] Table 7
[0087]
[0088]
[0089] The preparation method of eszopiclone orally disintegrating tablets in the above-mentioned embodiments 1-9 comprises the following steps:
[0090] S1. Put the eszopiclone and auxiliary materials into a universal pulverizer for 30-50 minutes and pulverize them to obtain powdery materials. Pass the eszopiclone through an 80-mesh sieve, sieve 3 times, and mix PVPP and mannitol 200 SD The mixture with MCC and stevioside are passed through a 30-mesh sieve, sieved for 3 times, and the eszopiclone and auxiliary materia...
Embodiment 10
[0097] (1) Verification test
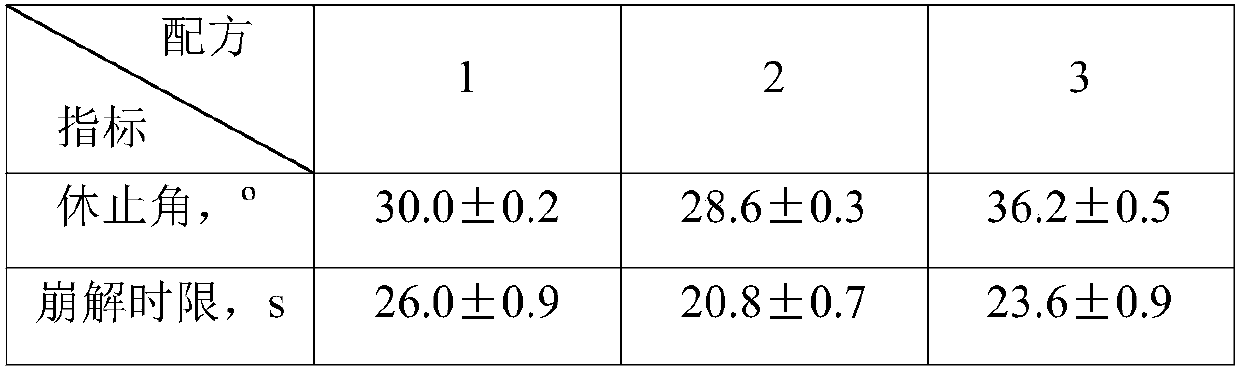
[0098] Three batches of samples were prepared according to the formula ratio of eszopiclone orally disintegrating tablets in Example 9, and the appearance, taste, tablet weight difference, hardness, disintegration time limit, and eszopiclone content were detected according to the relevant quality requirements of orally disintegrating tablets. , the results are shown in Table 9.
[0099] Table 9 Quality evaluation of 3 batches of eszopiclone orally disintegrating tablets
[0100]
[0101] (2) Methodological investigation
[0102] In order to further investigate the performance of the prepared eszopiclone orally disintegrating tablets, specificity inspection, linear relationship inspection, precision test, stability test, repeatability test and sample recovery test were carried out respectively.
[0103] The experimental results show that: the specificity test shows that the excipients in the sample do not interfere with the main peak of eszop...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com