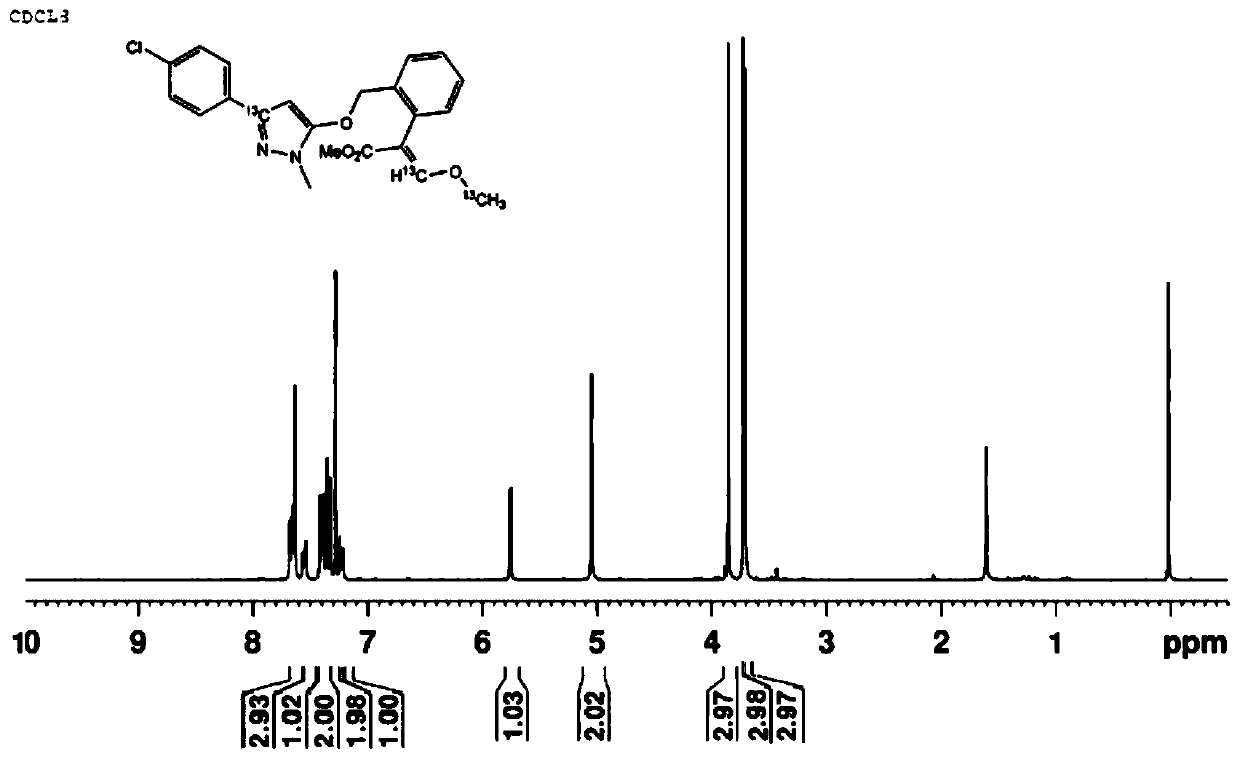
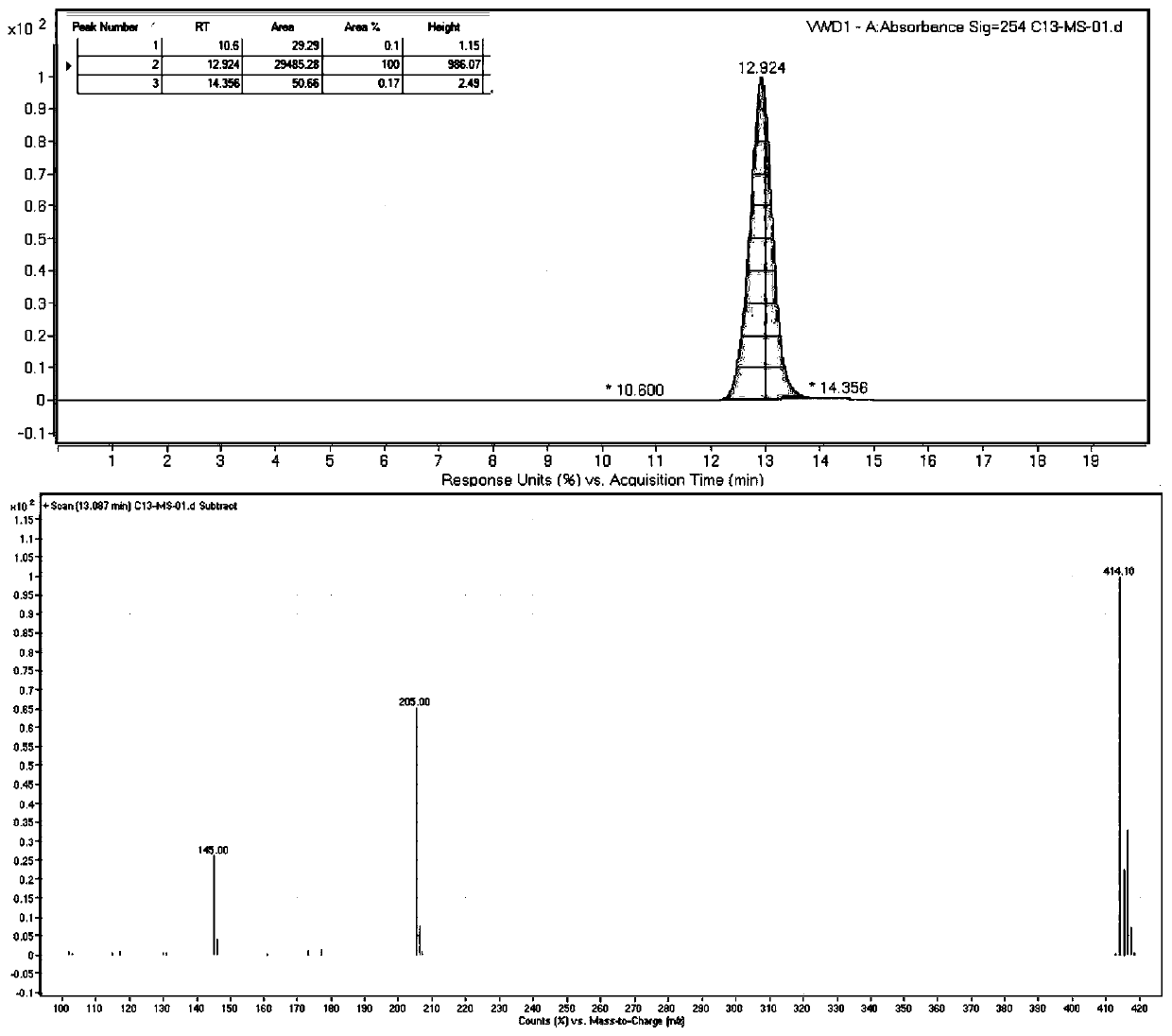
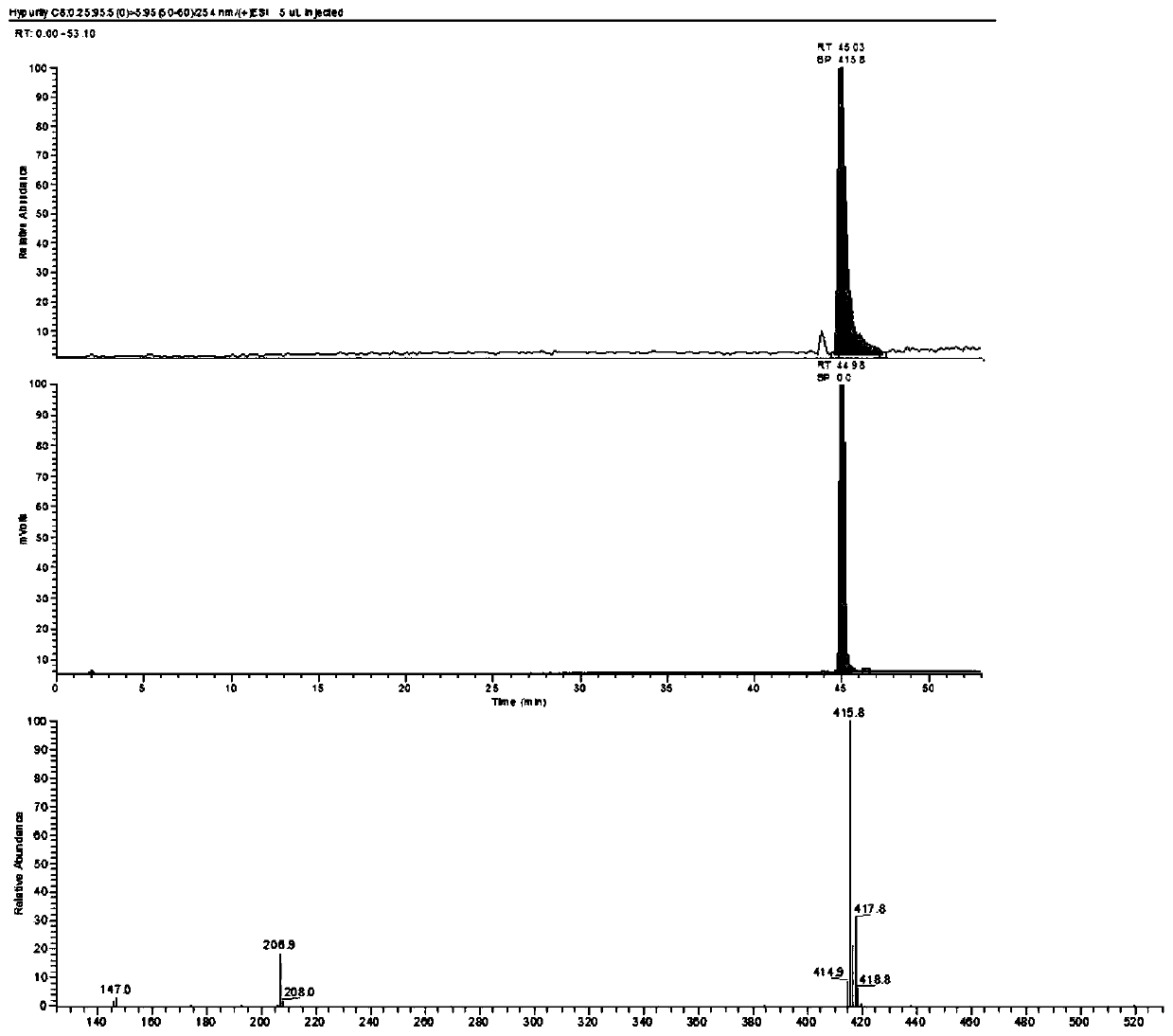
13 c-labeled pyraclostrobin and its synthesis method
A pyraclostrobin and labeling technology is applied in the direction of organic chemical methods, chemical instruments and methods, and fungicides. It can solve the problems of many impurities, complex synthesis processes, and difficult purification, and achieves easy separation and purification, simple operation process, and Effect of High Atom Utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] The present invention provides a 13 The synthetic method of the pyraclostrobin of C mark, comprises the steps:
[0040] Step (a): using chlorobenzene as a raw material to obtain the first product through acylation reaction and condensation ring closure reaction;
[0041] Step (b): methyl o-toluene acetate undergoes condensation reaction, methylation reaction and bromination reaction to obtain the second product;
[0042] Step (c): the first product and the second product are reacted in an alkaline environment to obtain 13 C-labeled pyraclostrobin.
[0043] Among them, the stable isotope 13 The C label is introduced by one or more of the acylation reaction in step (a), the condensation reaction in step (b), or the methylation reaction, such as a single-site stable isotope 13 The C label can be introduced by acylation in step (a), by condensation in step (b), or by methylation in step (b); stable isotopes at both sites 13 The C label can be introduced by an acylation r...
Embodiment 1
[0091] This embodiment provides a 13 A preparation method for C-labeled pyraclostrobin, the method comprising the following steps:
[0092] (1) Preparation of 3-(4-chlorophenyl)-1-methyl-1H-5-pyrazolol (I-5)
[0093] Under the protection of nitrogen, aluminum trichloride (5.10g, 7.58mmol) and chlorobenzene (7.0mL, 69.40mmol) were added to a 150mL stuffy jar, and added by syringe at room temperature 13 C-labeled acetyl chloride (5.0 g, 62.89 mmol) was post-blocked and stirred at 55 °C for 6 h. After cooling, the reaction solution was quenched in ice water, extracted, and concentrated to obtain p-chloroacetophenone (8.90 g, yield 82.40%) as light yellow oil. The tetrahydrofuran (50mL) solution of p-chloroacetophenone (5.26g, 33.72mmol) was respectively charged into the three-necked flask protected by nitrogen, and NaHMDS (2M in THF, 25.3mL, 50.58mmol) was added under the ice-salt bath and stirred for 0.5h, and then Dimethyl carbonate (3.1 mL, 37.09 mmol) was added and the mix...
Embodiment 2
[0099] 13 A preparation method for C-labeled pyraclostrobin, the method comprising the following steps:
[0100] (1) Preparation of 3-(4-chlorophenyl)-1-methyl-1H-5-pyrazolol (I-5)
[0101] Under the protection of nitrogen, aluminum trichloride (5.10g, 7.58mmol) and chlorobenzene (7.0mL, 69.4mmol) were added in a 150mL stuffy jar, and acetyl chloride (5.00g, 62.89mmol) was added through a syringe at room temperature to seal, And stirred at 55°C for 6h. After cooling, the reaction solution was quenched in ice water, extracted, and concentrated to obtain p-chloroacetophenone (8.9 g, yield 82.4%) as light yellow oil. NaH (60%, 2.72g, 68.12mmol), tetrahydrofuran (50mL) and dimethyl carbonate (3.1mL, 37.09mmol) were respectively charged into a three-neck flask protected by nitrogen. The mixture was heated to reflux and stirred for 30 min, then a tetrahydrofuran solution of p-chloroacetophenone (5.26 g, 33.72 mmol) was added dropwise within 15 min, continued to stir for 6 h and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com