Application of corynebacterium diphtheroid as immune adjuvant in poultry oil emulsion inactivated vaccine
A coryneform bacillus and inactivated vaccine technology, applied in the field of microorganisms, can solve the problems of impossible use in animal vaccines, human and animal pathogenicity, and limited stimulating effect of adjuvants, and achieves high safety, fast proliferation, Low loss of active ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
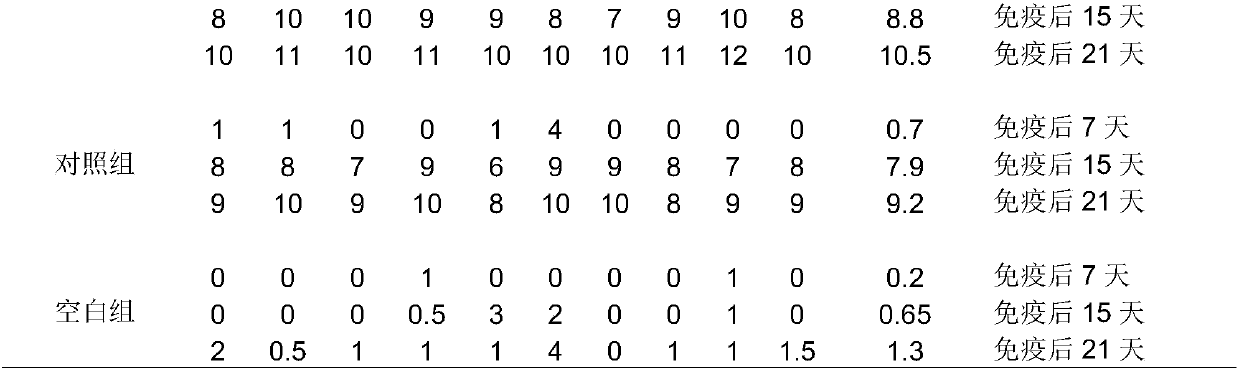
[0026] Embodiment 1: the preparation of Newcastle disease+H9 subtype avian influenza dual oil emulsion inactivated vaccine (abbreviated as new stream dual vaccine)
[0027] (1) Sterilization and quantification of adjuvant samples
[0028] Diphtheriae-like corynebacterium adjuvant product powder sample 1g, dissolved in 40mL of normal saline to prepare 25mg / mL concentration of adjuvant solution, the adjuvant solution was routinely sterilized and stored at 4°C for later use.
[0029] (2) Preparation of antigen aqueous phase:
[0030] ① Antigen water phase of control group without adjuvant: take 94mL inactivated Newcastle disease + H9 mixed antigen (Newcastle disease antigen (ND antigen for short): H9 subtype avian influenza antigen = 1:1 mixed, Newcastle disease antigen and H9 subtype Type avian influenza antigens are prepared from chicken embryos), add 2mL sterilized normal saline under aseptic conditions, then add 4mL sterilized TWEEN80, shake or stir until TWEEN80 is complete...
Embodiment 2
[0056] Embodiment 2: the preparation of H5 subtype avian influenza (D7 strain) oil emulsion inactivated vaccine
[0057] (1) Sterilization and quantification of adjuvant samples
[0058] Diphtheriae-like corynebacterium adjuvant product powder sample 1g, dissolved in 40mL of normal saline to prepare adjuvant solution with a concentration of 25mg / mL, the adjuvant solution was subjected to routine sterilization treatment and stored at 4°C for later use.
[0059] (2) Preparation of antigen aqueous phase:
[0060] ①Aqueous phase of antigen in control group without adjuvant: take 94mL inactivated antigen of H5 subtype avian influenza D7 strain, add 2mL sterilized normal saline under sterile conditions, then add 4mL sterilized TWEEN80, shake or stir until TWEEN80 is completely dissolved , which is the antigen aqueous phase of the control group without adjuvant (the total amount is 100 mL).
[0061] ② Antigen aqueous phase of adjuvant group (final concentration of adjuvant: 0.5 mg / ...
Embodiment 3
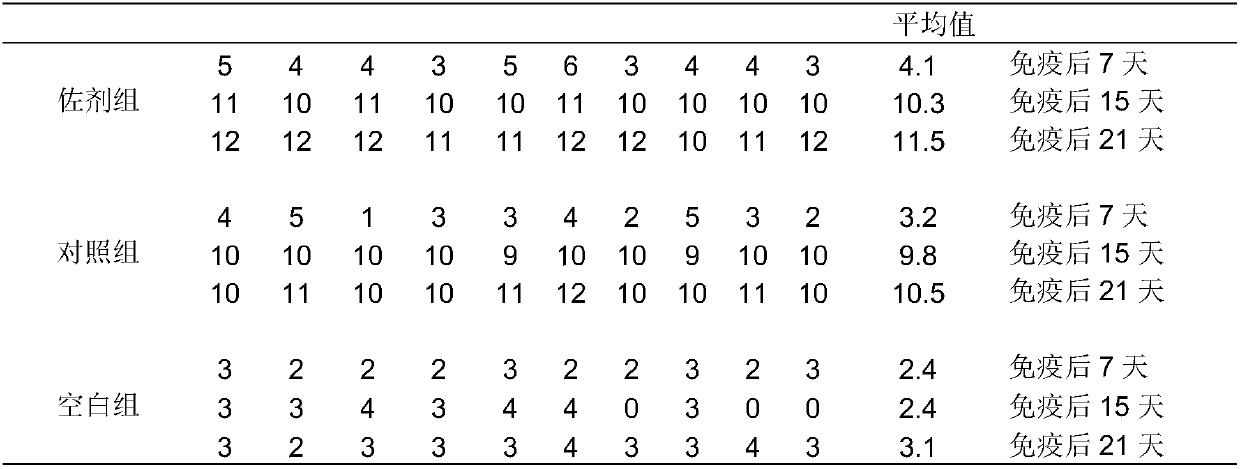
[0086] Embodiment 3: the preparation of H5 subtype avian influenza (D7+rD8 strain) divalent oil emulsion inactivated vaccine
[0087] (1) Sterilization and quantification of adjuvant samples
[0088] Diphtheriae-like corynebacterium adjuvant product powder sample 1g, dissolved in 40mL of normal saline to prepare 25mg / mL concentration of adjuvant solution, the adjuvant solution was routinely sterilized and stored at 4°C for later use.
[0089] (2) Preparation of antigen aqueous phase:
[0090]① Antigen aqueous phase of control group without adjuvant: take 94mL of inactivated mixed antigen containing H5 subtype avian influenza D7 strain and rD8 strain, add 2mL sterilized normal saline under sterile conditions, then add 4mL sterilized TWEEN80, shake or After stirring until TWEEN80 is completely dissolved, it becomes the antigen aqueous phase of the control group without adjuvant (the total amount is 100 mL).
[0091] ② Aqueous phase of antigen in adjuvant group (final concentra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com