Separation and purification method of high-purity moxidectin
A technology for the separation and purification of moxidectin, which is applied in the field of antibiotic separation and purification, can solve the problems of complex separation process, large amount of solvent, and high cost of moxidectin separation, and achieve good extraction ability, improved purification effect, and short phase separation time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
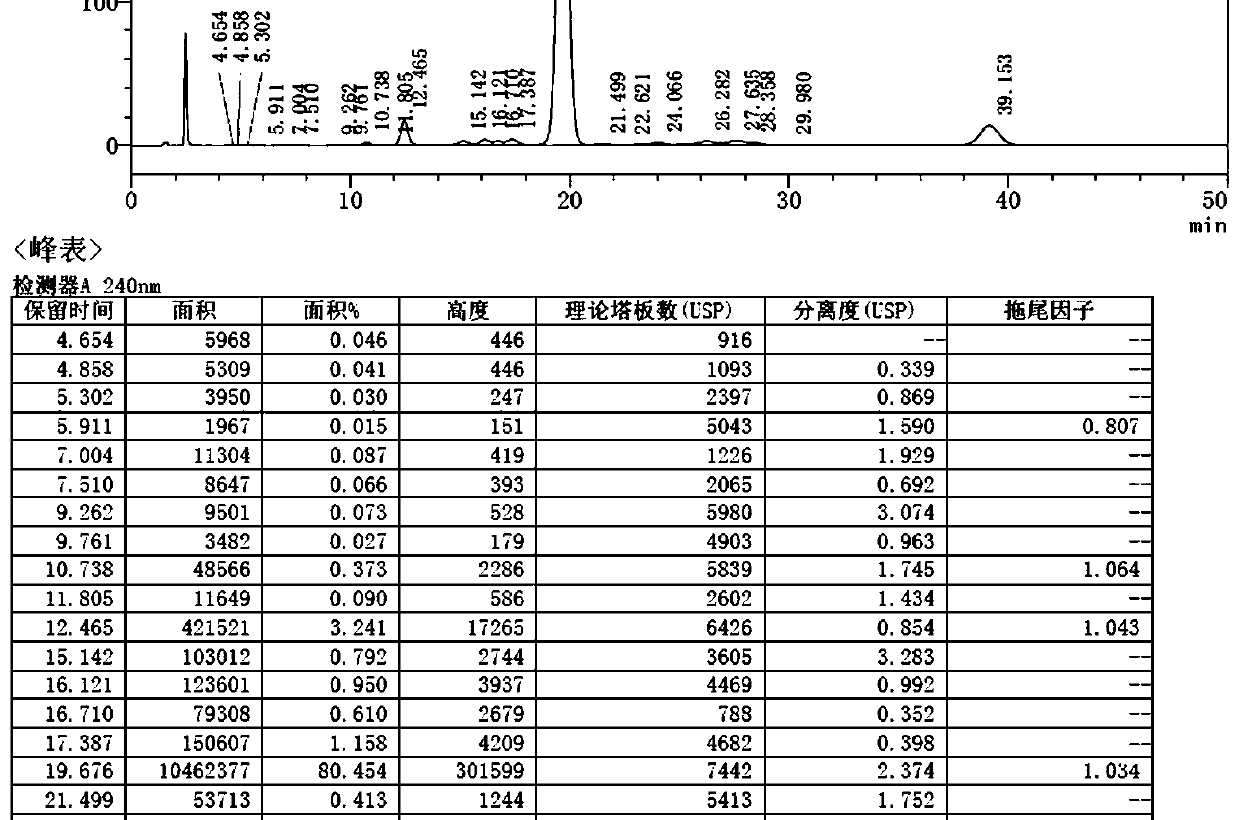
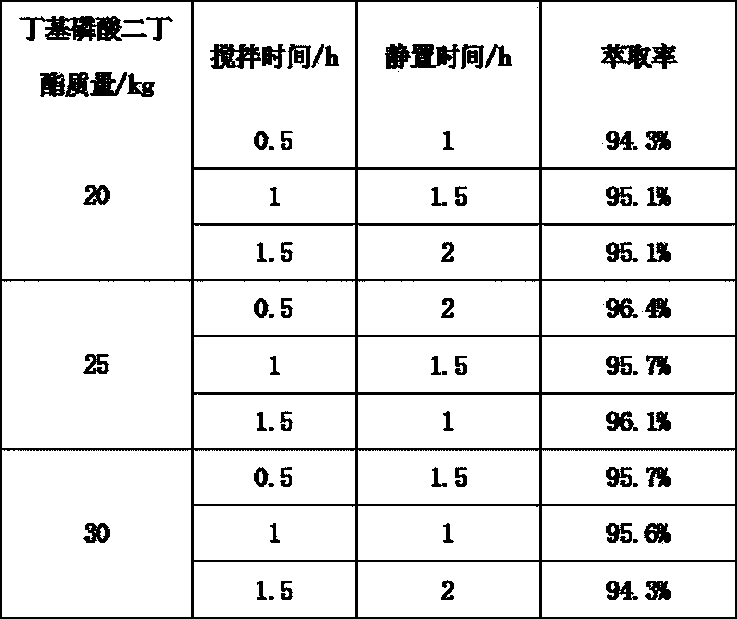
[0035] The fermentation broth produced by the streptomycin fermentation is separated from the solid and liquid, and the nimoxine concentrate is obtained after leaching, and the methanol solution of moximectin (MX-4) is obtained by the protection, oxidation, deprotection and amination reaction. 34.5kg, content 12.3%, purity 80.6%, pump the above solution into a stainless steel reactor, add MX-4 methanol solution equivalent to 40±1 times the volume of 68-72% methanol / acetonitrile / ethanol aqueous mobile phase to match The column liquid was prepared, and the titer was 21527μg / mL.
[0036] Take the mobile phase to equilibrate the preparative column (model: DAC-100 chromatography packing: ODS-C18) for 20-25 minutes at a flow rate of 2L / min.
[0037] Add 20 kg of methyl methacrylate to the methanol solution of moximectin and heat for 1 h.
[0038] Sampling: open the system equipment monitor, select the appropriate online filter, set the sampling mode to automatic, set the feed flow rate to...
Embodiment 2
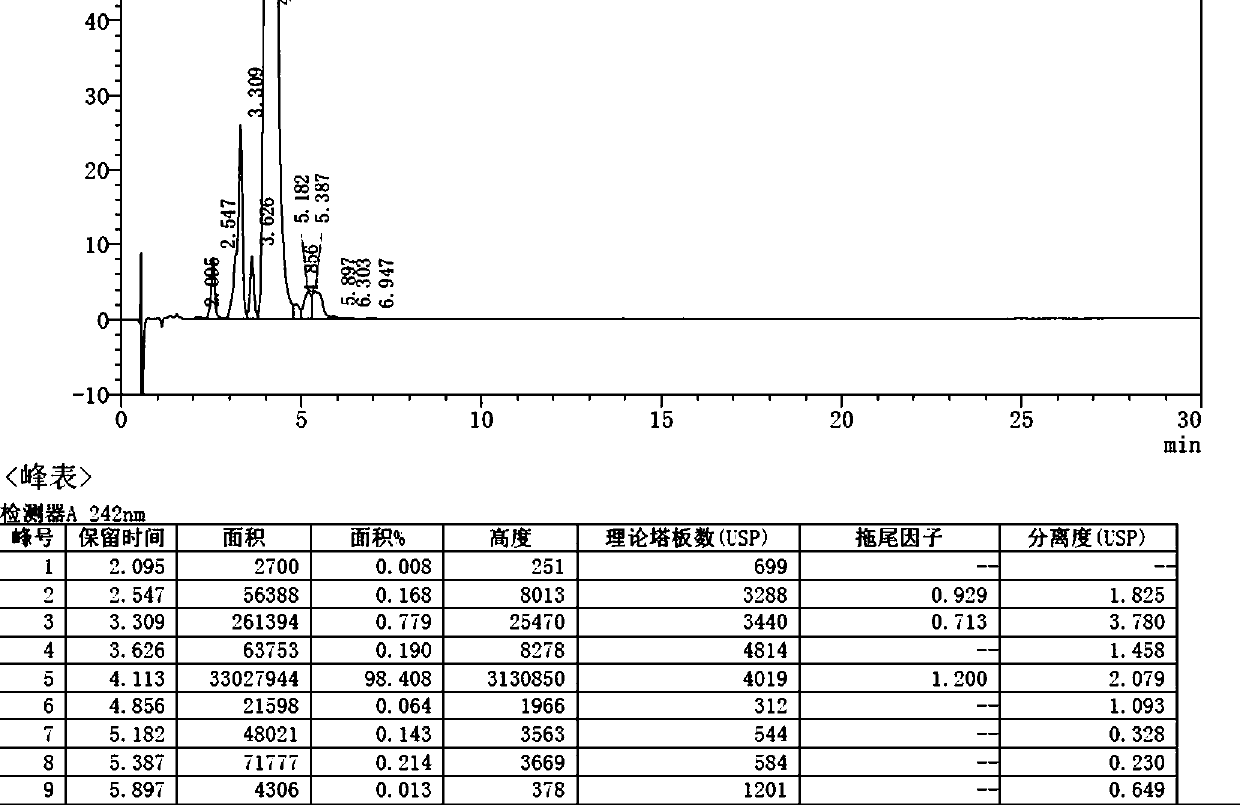
[0045] The fermentation broth produced by the streptomycin fermentation is separated from the solid and liquid, and the nimoxetine concentrate is obtained after leaching, and the methanol solution of moximectin is obtained by the protection, oxidation, deprotection and amination reaction (conventional reaction). MX-4) 31.3kg, the content is 15.7%, the purity is 84.7%, the above solution is pumped into a stainless steel reactor, and the volume of the MX-4 methanol solution is 40±1 times the volume of 68-72% methanol / acetonitrile / The mobile phase of the ethanol aqueous solution was mixed into the upper column liquid, and the titer was 18828μg / mL.
[0046] Take the mobile phase to equilibrate the preparative column (model: DAC-100 chromatography packing: ODS-C18) for 20-25 minutes at a flow rate of 2L / min.
[0047] 21kg methyl methacrylate was added to the methanol solution of moximectin and heated for 1.5h.
[0048] Sampling: open the system equipment monitor, select the appropriate ...
Embodiment 3
[0055] The fermentation broth produced by the streptomycin fermentation is separated from the solid and liquid, and the nimoxetine concentrate is obtained after leaching, and the methanol solution of moximectin is obtained by the protection, oxidation, deprotection and amination reaction (conventional reaction). MX-4) 28.5kg, content 15.8%, purity 80.8%, pump the above solution into a stainless steel reactor, add MX-4 methanol solution equivalent to 40±1 times the volume of 68-72% methanol / acetonitrile / The mobile phase of the ethanol aqueous solution was mixed into the upper column liquid, and the titer was 22214μg / mL by sampling.
[0056] Take the mobile phase to equilibrate the preparative column (model: DAC-100 chromatography packing: ODS-C18) for 20-25 minutes at a flow rate of 2L / min.
[0057] 22kg of methyl methacrylate was added to the methanol solution of moximectin and heated for 2h.
[0058] Sampling: open the system equipment monitor, select the appropriate online filter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com