Gas chromatography-mass spectrometry detection method for chloromethyl methyl ether residues in raw materials
A gas chromatography-mass spectrometry and chloromethyl methyl ether technology, which is applied in the field of gas chromatography-mass spectrometry detection of chloromethyl methyl ether residues in raw materials, can solve the cumbersome sample preparation process and the low precision and accuracy of quantitative analysis , time-consuming and other issues, to achieve the effect of simplifying the sample preparation process, good sensitivity, and improving precision and accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The specificity research of chloromethyl methyl ether residual determination in embodiment 1 crude drug:
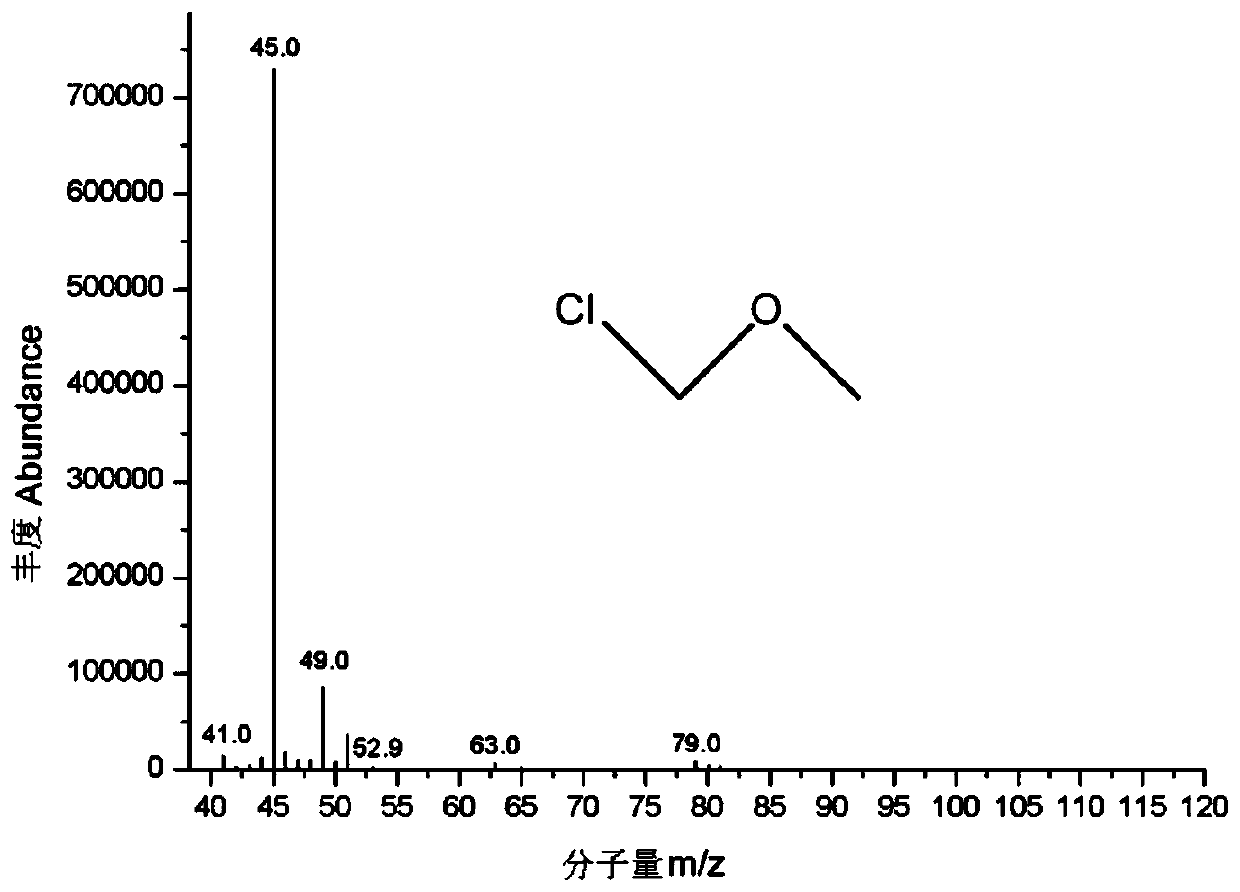
[0032] Precisely weigh about 100mg of chloromethyl methyl ether (CMME) into a 100mL volumetric flask containing about 10mL of dichloromethane, dilute to the mark with dichloromethane, shake well, and mark it as CMME-DCM solution. Chloromethyl methyl ether is stable in dichloromethane.
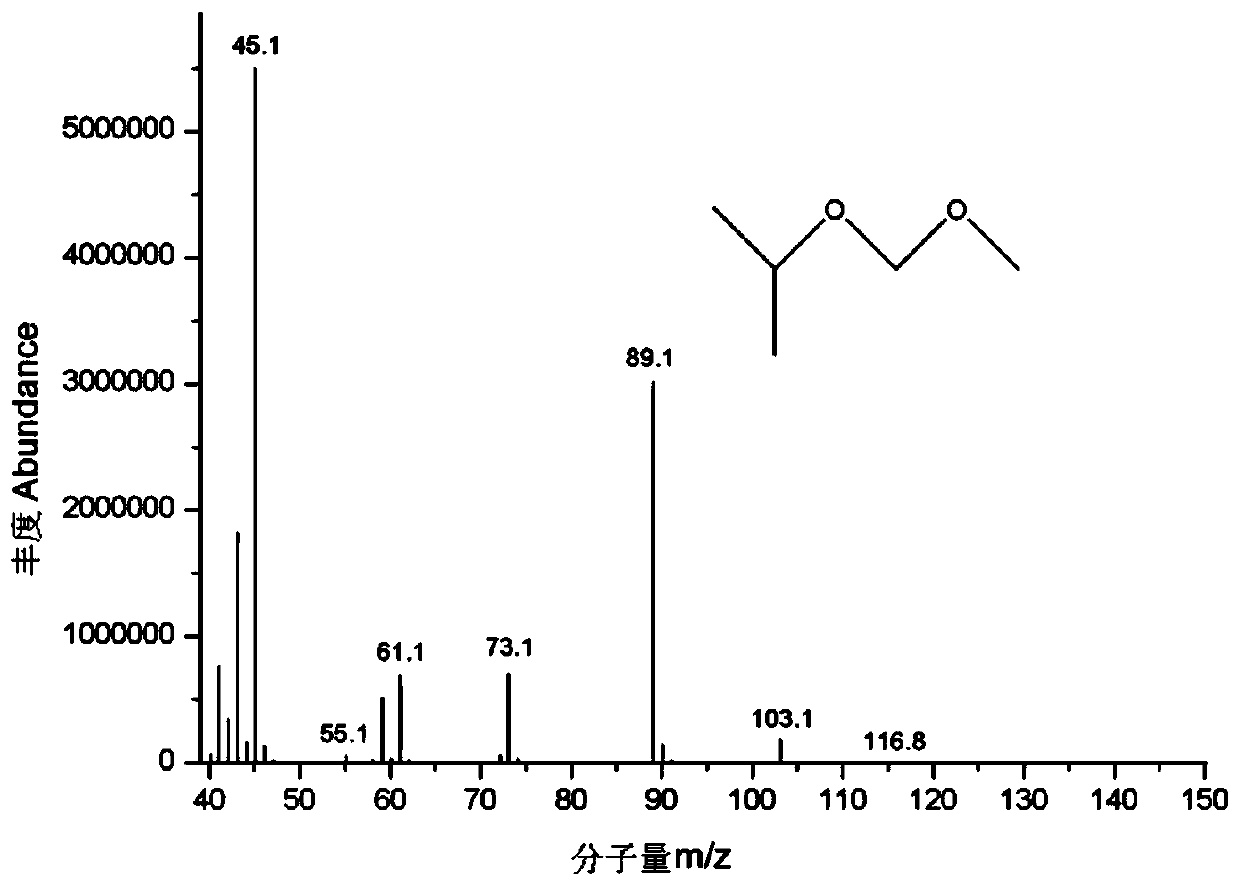
[0033] Precisely weigh about 100mg of chloromethyl methyl ether into a 100mL volumetric flask containing about 10mL of isopropanol, dilute to the mark with isopropanol, shake well, and mark it as CMME-IPA solution. Chloromethyl methyl ether reacts with isopropanol in isopropanol solution, and the reaction is as follows:
[0034]
[0035] Carry out GC-MS analysis to CMME-DCM solution and CMME-IPA solution respectively, method condition:
[0036] The chromatographic column adopts DB-624 capillary gas chromatography column, the chromatographic column length is 30m, the inner diame...
Embodiment 2
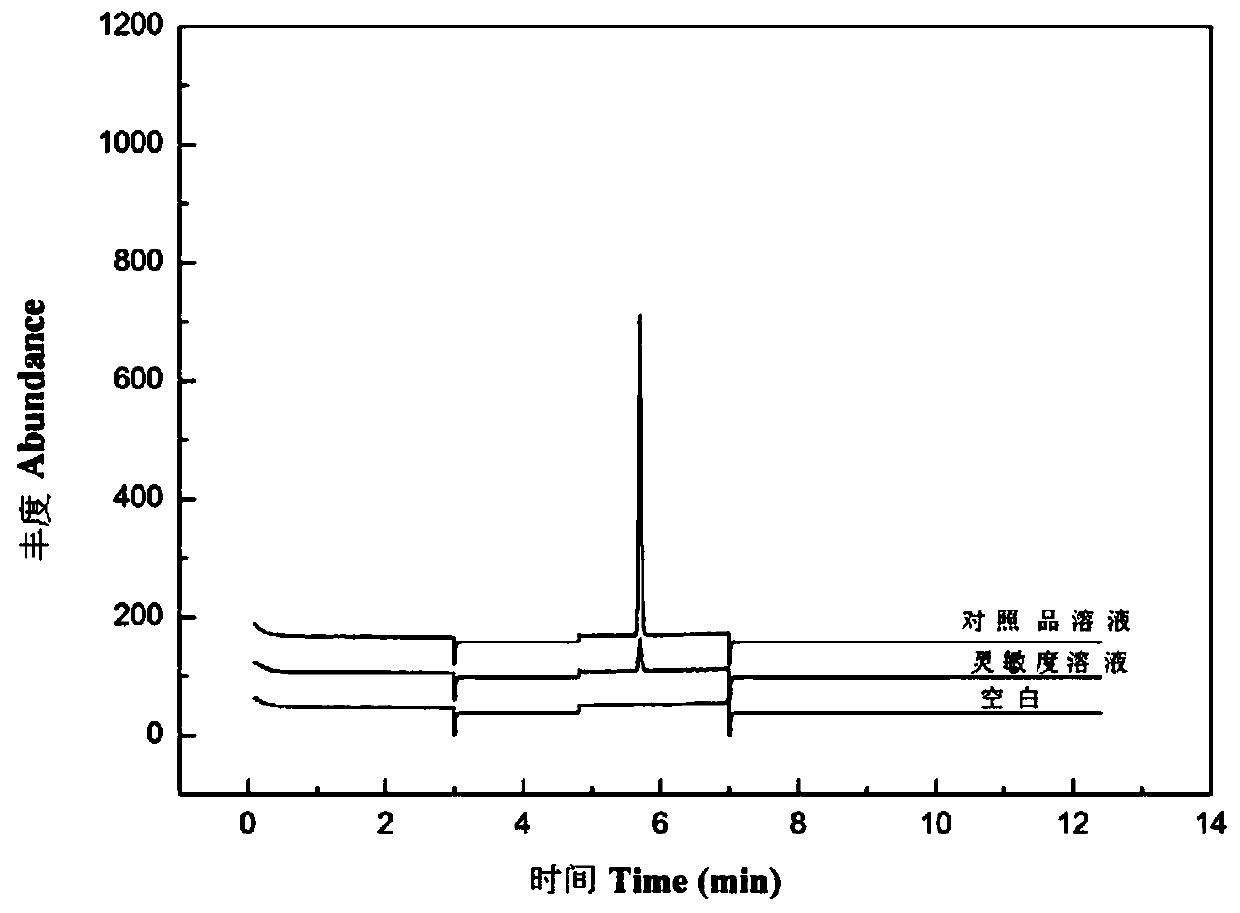
[0045] Sensitivity, precision, linearity, accuracy and stability research of chloromethyl methyl ether residual determination in embodiment 2 crude drug:
[0046] Accurately weigh about 100mg of chloromethyl methyl ether into a 100mL volumetric flask containing about 10mL of isopropanol, dilute to the mark with isopropanol and shake well, accurately pipette 0.6mL of the solution into a 100mL volumetric flask, Dilute the volume of propanol to the mark, shake well, and mark it as the reference substance stock solution.
[0047] GC-MS analysis method conditions:
[0048] The chromatographic column adopts DB-624 capillary gas chromatography column, the chromatographic column length is 30m, the inner diameter is 0.32mm, and the film thickness is 1.8μm. ;
[0049] Helium was used as the carrier gas, and the carrier gas flow rate was 1.5mL / min;
[0050] Direct injection was adopted, the injection volume was 1 μL, and the temperature of the injection port was 200°C;
[0051] Using...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com