Preparation method of lithium hexafluorophosphate
A technology of lithium hexafluorophosphate and lithium fluoride, applied in lithium hexafluorophosphate, chemical instruments and methods, lithium compounds, etc., can solve the problems of complex processing technology and difficult product purification, and achieve the effect of simple subsequent processing, resource saving and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
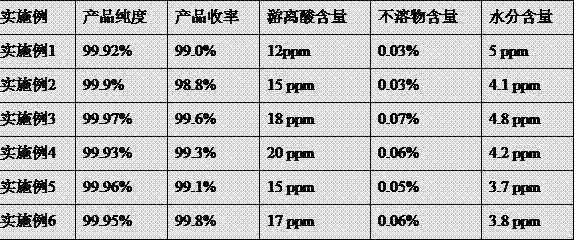
Examples
Embodiment 1
[0040] In a dry airtight atmosphere with a water content of less than 10ppm, the reactor was filled with anhydrous CO 2 After the gas has been replaced many times, lithium fluoride and liquid carbon dioxide are added to the reaction vessel at a mass ratio of 1:8 and stirred, and the temperature in the reactor is always kept lower than that of CO 2 critical temperature, and a certain pressure to maintain CO 2 In this embodiment, the temperature in the reactor is maintained at 20°C lower than room temperature, and the pressure is 8MPa, and then PF is added to the reaction vessel 5 Gas, the molar ratio of lithium fluoride and phosphorus pentafluoride added is 1:1.5, and then the reaction temperature is controlled at -12°C and the pressure is 5MPa. After the reaction is completed, the temperature of the reactor is raised to room temperature (20°C). The pressure is reduced to normal pressure (1atm), and the excess phosphorus pentafluoride gas and CO are discharged 2 gas, and the ...
Embodiment 2
[0042] In a dry airtight atmosphere with a water content of less than 10ppm, the reactor was filled with anhydrous CO 2 After the gas has been replaced many times, lithium fluoride and liquid carbon dioxide are added to the reaction vessel at a mass ratio of 1:20 and stirred, and the temperature in the reactor is always kept lower than that of CO 2 critical temperature, and a certain pressure to maintain CO 2 liquid state, and then add PF to the reaction vessel 5 Gas, the molar ratio of lithium fluoride to phosphorus pentafluoride added is 1:1.03, the reaction temperature is controlled at 5°C, and the pressure is 6MPa. After the reaction is completed, the temperature of the reactor is raised to room temperature, and the pressure is reduced to normal pressure. Exhaust excess phosphorus pentafluoride gas and CO 2 gas, and the resulting lithium hexafluorophosphate product was collected.
Embodiment 3
[0044] In a dry airtight atmosphere with a water content of less than 10ppm, the reactor was filled with anhydrous CO 2 After the gas has been replaced many times, lithium fluoride and liquid carbon dioxide are added to the reaction vessel at a mass ratio of 1:45 and stirred, and the temperature in the reactor is always kept lower than that of CO 2 critical temperature, and a certain pressure to maintain CO 2 liquid state, and then add PF to the reaction vessel 5 Gas, the molar ratio of lithium fluoride to phosphorus pentafluoride added is 1:6, the reaction temperature is controlled at 26°C, and the pressure is 10MPa. to room temperature), discharge excess phosphorus pentafluoride gas and CO 2 gas, and the resulting lithium hexafluorophosphate product was collected.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com