Method for preparing 1,1,1,2,3-pentafluoropropane
A technology of pentafluoropropane and pentafluoropropene, which is applied in the field of preparation of chemical products, can solve the problems of low reaction efficiency and high energy consumption, and achieve the effects of prolonging the operation cycle, life extension and stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
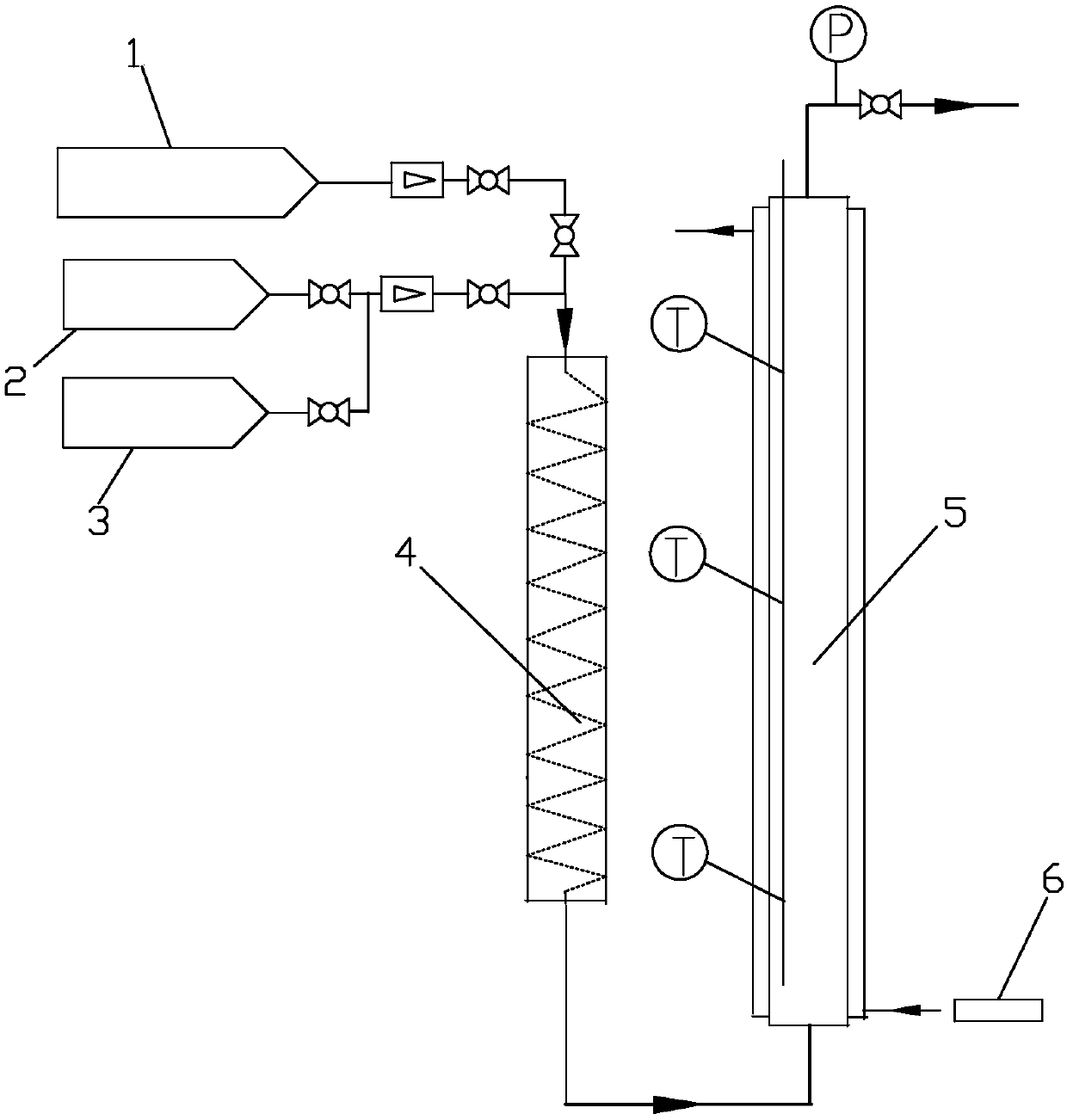
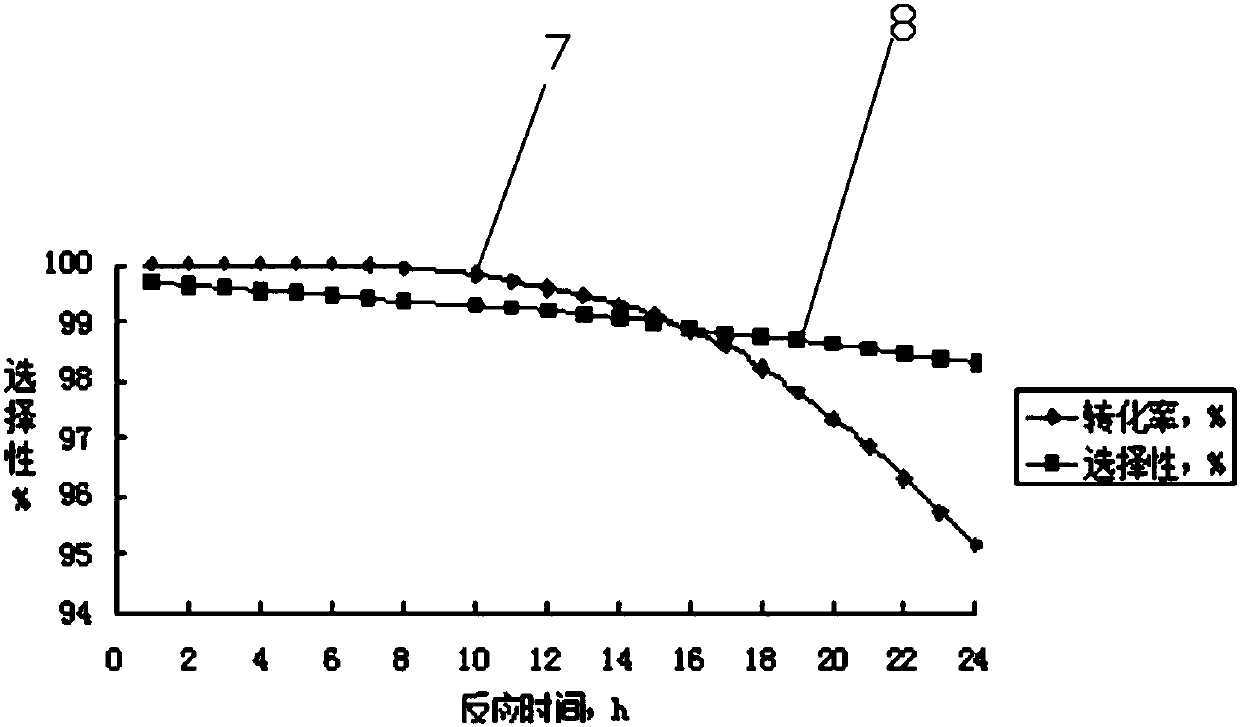
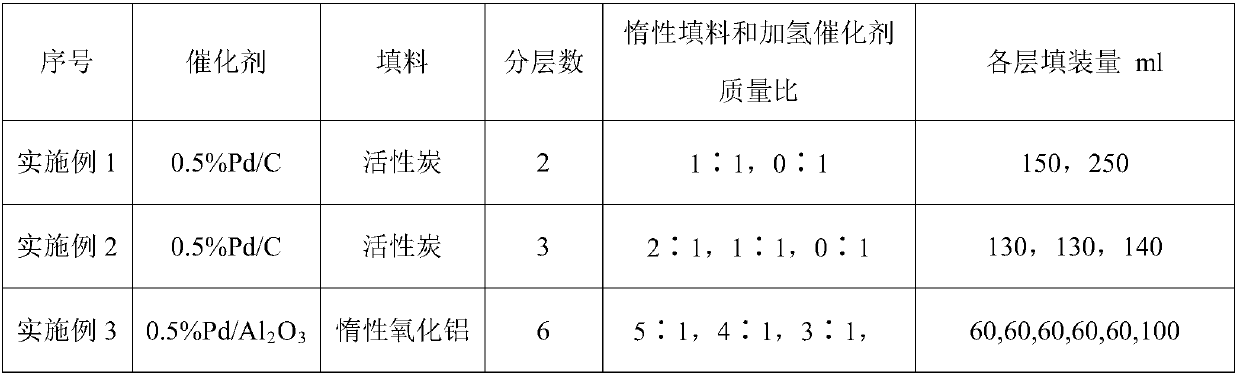
[0042] Use the tubular reactor 5 that is made of stainless steel, its inner diameter is 2.5cm, length is 150cm, built-in temperature jacket, external cooling jacket, fill 586g (being 400cm 3 ) of an inert filler and a hydrogenation catalyst mixture in a suitable proportion, the catalyst layer is dried with nitrogen and activated with hydrogen before the reaction, and then the hydrogenation reaction is carried out. After mixing hydrogen at 32mol / h and 1,1,1,2,3-pentafluoropropene at a feed rate of 4mol / h, it first enters the preheater 4 for preheating; then from the lower end of the heat exchange fixed bed reactor 5 (that is, the catalyst end with low active component content) enters, and the temperature of the mixed gas entering the reactor 5 is controlled to be 85-90°C, and the reaction pressure is 0.45-0.50MPa. During the continuous reaction of 1000 hours, the highest reaction temperature of the catalyst bed is 150~156℃, and the reaction temperature is stable. A conversion ...
Embodiment 2
[0044] Use the tubular reactor 5 that is made of stainless steel, its internal diameter is 2.5cm, length is 150cm, built-in temperature jacket, external cooling jacket, fill 586g (being 400cm 3 ) of an inert filler and a hydrogenation catalyst mixture in a suitable proportion, the catalyst layer is dried with nitrogen and activated with hydrogen before the reaction, and then the hydrogenation reaction is carried out. After mixing hydrogen at 20mol / h and 1,1,1,2,3-pentafluoropropene at a feed rate of 4mol / h, it first enters the preheater 4 for preheating; then from the lower end of the heat exchange fixed bed reactor 5 (that is, the catalyst end with low active component content) enters, and the temperature of the mixed gas entering the reactor 5 is controlled to be 75-80°C, and the reaction pressure is 0.45-0.50MPa. During the continuous reaction of 1000 hours, the highest reaction temperature of the catalyst bed is 165~169℃, and the reaction temperature is stable. A conversi...
Embodiment 3
[0046] Use the tubular reactor 5 that is made of stainless steel, its internal diameter is 2.5cm, length is 150cm, built-in temperature jacket, external cooling jacket, fill 586g (being 400cm 3 ) of an inert filler and a hydrogenation catalyst mixture in a suitable proportion, the catalyst layer is dried with nitrogen and activated with hydrogen before the reaction, and then the hydrogenation reaction is carried out. After mixing hydrogen at 6mol / h and 1,1,1,2,3-pentafluoropropene at a feed rate of 4mol / h, it first enters the preheater 4 for preheating; then from the lower end of the heat exchange fixed bed reactor 5 (that is, the catalyst end with low active component content) enters, the temperature of the mixed gas entering the reactor 5 is controlled to be 50-55°C, and the reaction pressure is 0.40-0.45MPa. During the continuous reaction of 1000 hours, the highest reaction temperature of the catalyst bed is 190~195℃, and the reaction temperature is stable. A 1,1,1,2,3-pen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com