Cu catalyst for hydrogen production by methanol steam reforming and preparation method and application thereof
A steam reforming and catalyst technology, applied in the direction of metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, heterogeneous catalyst chemical elements, etc., can solve the problem of unsatisfactory industrialization of stability and catalyst deactivation , grain growth and other issues, to achieve good catalytic activity, good activity and stability, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
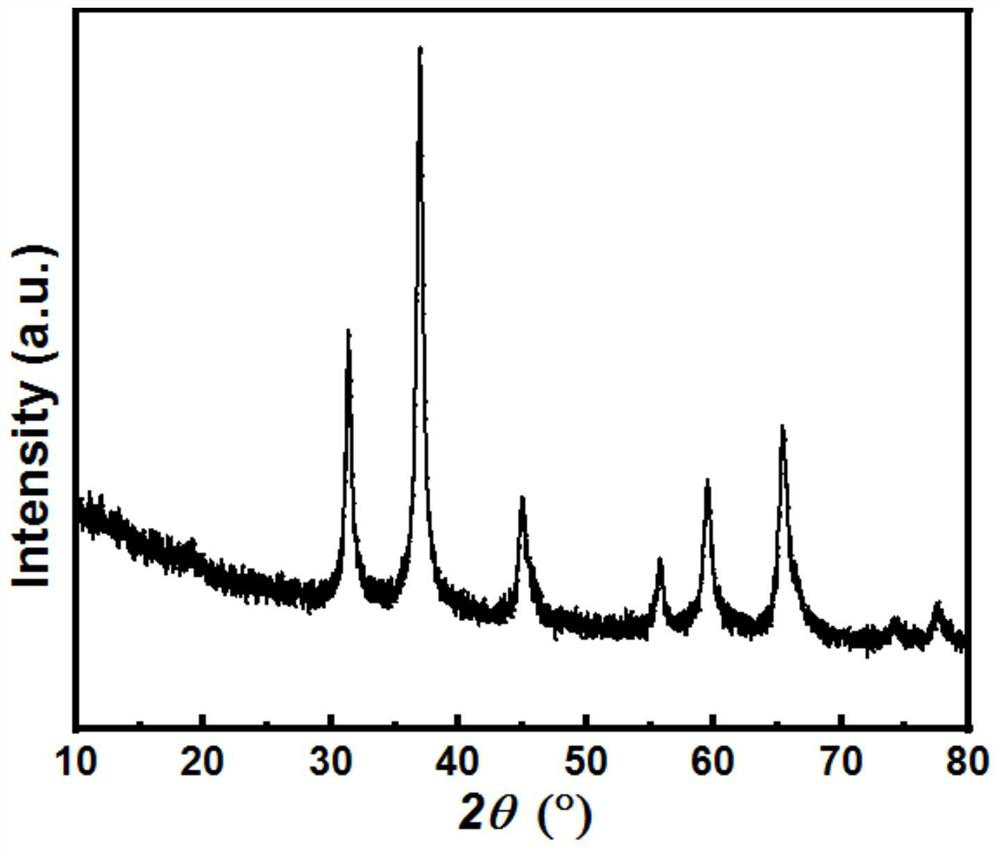
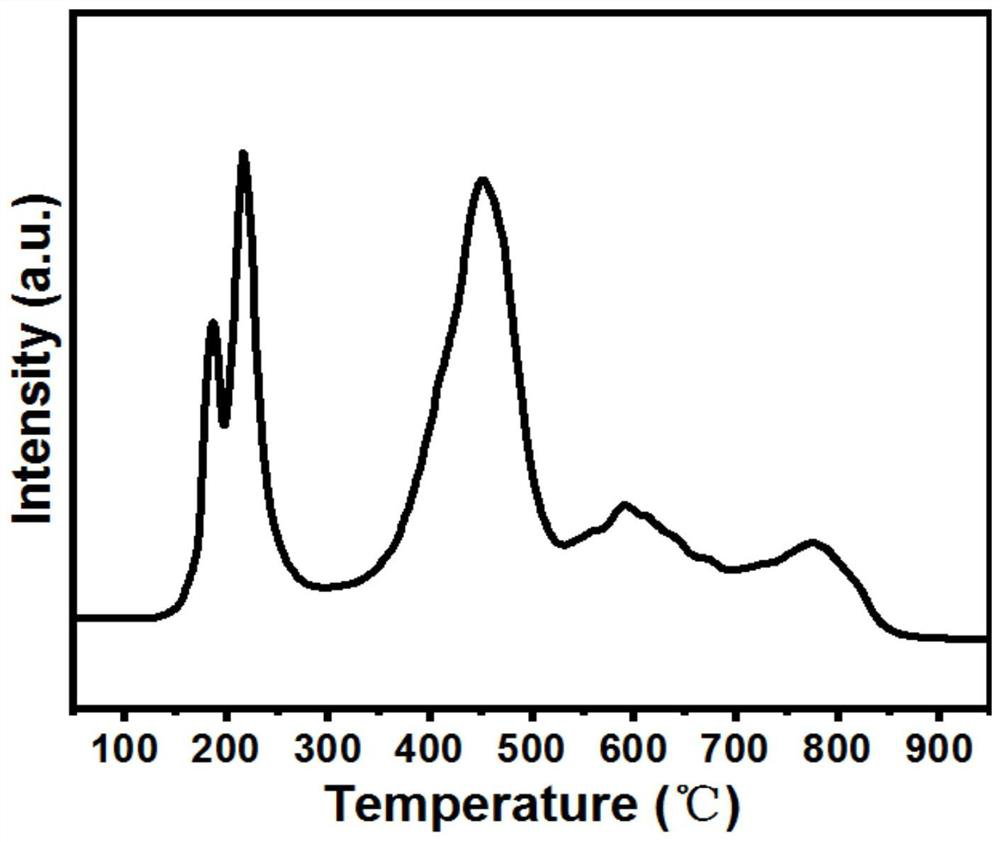
[0038] Accurately weigh 5.95 grams of CuCl 2 2H 2 O, 51.10 g Al(NO 3 ) 3 9H 2 O and 1.25 g of 50% Mn(NO 3 ) 2 solution, add deionized water to make a 200ml mixed solution; accurately weigh 25.73 grams of Na 2 CO 3, adding deionized water to form a 200ml mixed solution. Through the injection pump, inject the two aqueous solutions obtained above into the reactor at a uniform speed, stir vigorously, age at a constant temperature of 70°C for 3 hours, then filter, dry the filter cake at 100°C, press into tablets, grind through a 40-mesh sieve, and sieve The powder is placed in a muffle furnace and fired at 850°C for 0h (that is, the muffle furnace starts from normal temperature, and the temperature is programmed to rise to 850°C at a rate of 3°C / min, and then the operation of maintaining a constant temperature (0h) is not performed, and the temperature is directly lowered to normal temperature. ). That is the catalyst, the mass fraction of each component is: CuO=27.94%, Al...
Embodiment 2
[0043] Accurately weigh 8.36 grams of Cu(NO 3 ) 2 ·3H 2 O, 24.21 g Al(NO 3 ) 3 9H 2 O, 2.48 grams of mass fraction are 50% Mn (NO 3 ) 2 solution and 0.48 g of Zr(NO 3 ) 4 5H2O, add 50% ethanol water solution to form a 200ml mixed solution, accurately weigh 6.63 grams of γ-Al 2 o 3 Put into the middle of the reactor; Accurately weigh 13.42 grams (NH 4 ) 2 CO 3 , adding a mass fraction of 50% aqueous ethanol to form a 200ml mixed solution. Pour the above-mentioned two solutions into the reactor at a constant speed through a sampling pump, stir vigorously, age at a constant temperature of 60°C for 6 hours, then filter, dry the filter cake at 120°C, press into tablets, grind through a 40-mesh sieve, and sieve The powder is placed in a muffle furnace and calcined at 900°C for 3 hours to obtain the catalyst. The mass fraction of each component is: CuO=27.52%, Al 2 o 3 = 69.73%, Mn oxide = 1.38%, ZrO 2 = 1.37%. The catalyst evaluation method is the same as in Example...
Embodiment 3
[0045] Accurately weigh 8.32 grams of Cu(NO 3 ) 2 ·3H 2 O, 48.42 grams of Al(NO 3 ) 3 9H 2 O and 3.08 g of 50% Mn(NO 3 ) 2 solution, add deionized water to make a 200ml mixed solution; accurately weigh 25.08 grams of Na 2 CO 3 , adding deionized water to form a 200ml mixed solution. Through the injection pump, inject the two aqueous solutions obtained above into the reactor at a uniform speed, stir vigorously, age at a constant temperature of 65°C for 3 hours, then filter, dry the filter cake at 120°C, press into tablets, grind through a 40-mesh sieve, and sieve The powder is placed in a muffle furnace and calcined at 800°C for 3 hours to obtain the catalyst. The mass fraction of each component is: CuO=27.54%, Al 2 o 3 = 65.83%, Mn oxide = 6.80%. The catalyst evaluation method is the same as in Example 1, and the reaction conditions and results are shown in Table 1.
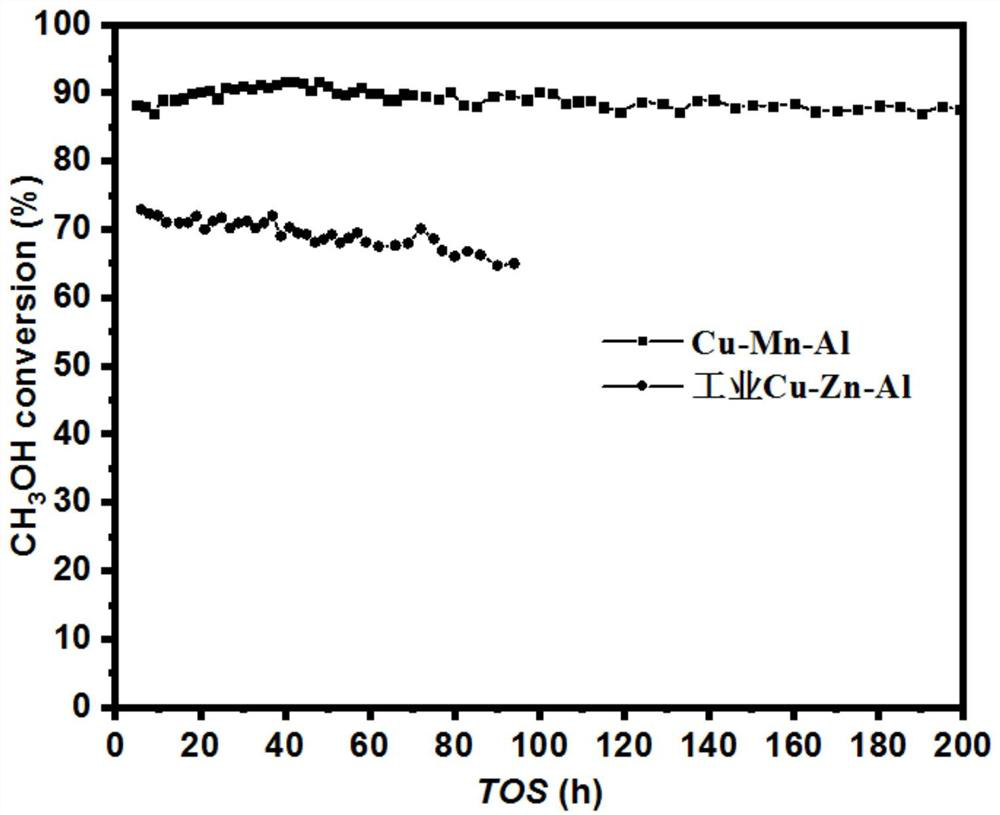
[0046] The above-mentioned synthesized catalyst is compared with the Cu-Zn-Al catalyst used in ind...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com