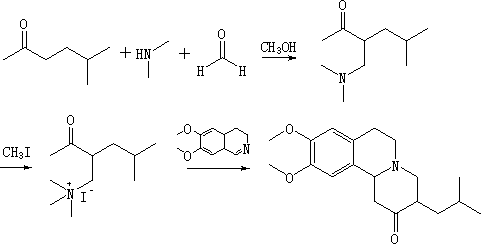
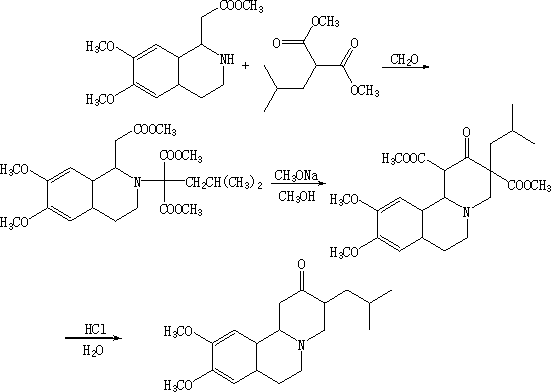
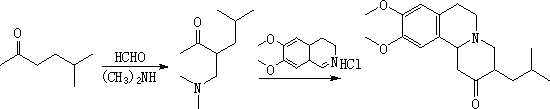
Synthetic method for tetrabenazine and intermediate of tetrabenazine
A synthesis method and technology of tetrabenazine are applied in the field of synthesis of tetrabenazine and intermediates thereof, and can solve the problems of difficult removal of impurities and poor purity, and achieve the effects of increased selectivity, readily available raw materials, and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0039] Preparation of 3-dimethylaminomethyl-5-methyl-2-hexanone (intermediate)
[0040]Add dimethylamine hydrochloride (41g, 0.5mol), paraformaldehyde (29.5g, 1mol), 5-methyl-2-hexanone (210ml, 1.5mol) into 500ml methanol, add 0.1N hydrochloric acid ( 12mol / L, 4.2ml, 0.05mol), heated to react. The reaction temperature was controlled at 55-60° C., and the reaction was carried out for 24 hours. Remove the solvent under reduced pressure, add 300ml of water, now under acidic conditions, extract the unreacted raw materials with n-hexane, adjust the pH value of the aqueous phase to about 7-9 with dilute sodium hydroxide solution, extract with 100ml of n-hexane, and then adjust the pH value of the aqueous phase to about 7-9, re-extract 100ml, repeat 5-6 times, combine the organic phases and concentrate under reduced pressure, and rectify the residue under reduced pressure, collect 2mmHg, 75±1°C fractions, and obtain 31.6g of refined intermediates, with a yield of 37.1% (GC %>98%). ...
example 2
[0042] Synthesis of Tetrabenazine
[0043] The intermediate (3-dimethylaminomethyl-5-methyl-2-hexanone, 30g, 0.175mol) and 6,7-dimethoxy-3,4-dihydroisoquinoline hydrochloride (39.8g, 0.175mol) was added to 300ml of water, a catalyst (TEBAC, 4.0g, 0.018mol, 0.1N) was added, and the reaction was heated. The temperature is controlled at 50-60°C, and the reaction time is 24h. Stop stirring, cool down to room temperature, filter the solid, and wash twice with 100 ml of water to obtain 41.5 g of crude tetrabenazine as an off-white solid, with a yield of 81.3%. Recrystallized from absolute ethanol to obtain 40.6 g of refined tetrabenazine, with a total yield of 73.3% and an HPLC purity of >99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com