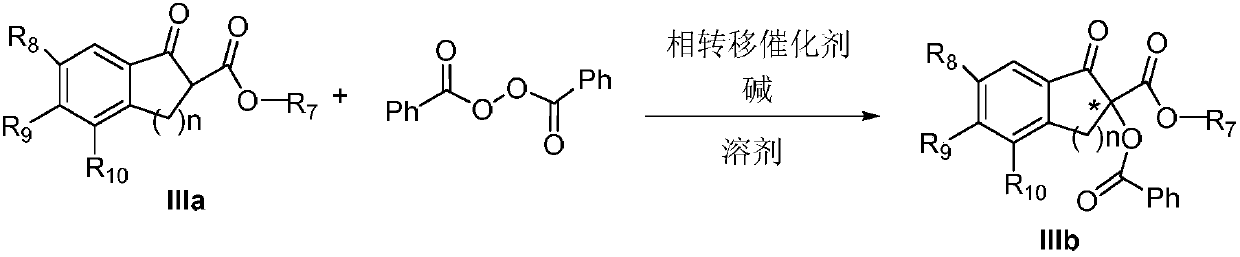
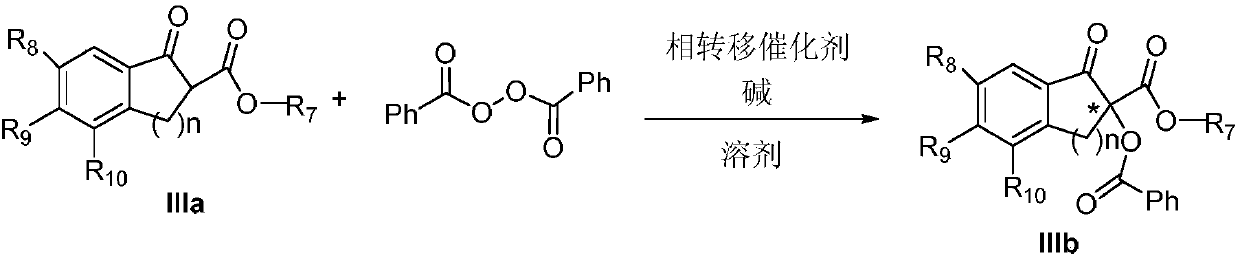
Method for achieving asymmetrical alpha-benzoylation through phase-transfer catalysis of beta-keto ester
A technology of phase transfer catalysis and phase transfer catalyst, which is applied in the directions of organic chemistry methods, chemical instruments and methods, carboxylate preparation, etc., to achieve the effect of easy separation and good catalytic effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of (S)-2-benzoyl-1-indanone-2-carboxylic acid methyl ester IIIb-1
[0025]
[0026] Weigh 0.1mmol 1-indanone-2-carboxylate adamantyl IIIa-1, add 5mol% phase transfer catalyst Ia-1, 0.15mol benzoyl peroxide (mass fraction 70%), put it into a 20mL single-port reaction tube, add K at a mass concentration of 30% 2 CO 3 Aqueous solution 0.5mL, 4mL toluene, stirred reaction at 25°C. After reacting for 24 hours, the mixture was extracted three times with ethyl acetate, washed three times with water, dried over anhydrous sodium sulfate, filtered, and spin-dried. The crude product was separated by column chromatography (petroleum ether: ethyl acetate = 25:1) to obtain the asymmetric benzoylated product IIIb-1 (31 mg, yield 72%, 43% ee); [α] D 25 110.6 (c 0.62, CHCl 3 ) 1 H NMR (400MHz, Chloroform-d) δ8.21–8.08(m,2H),7.88(d,J=7.6Hz,1H),7.73–7.66(m,1H),7.60(d,J=7.4Hz, 1H), 7.54–7.42(m, 4H), 4.14(d, J=17.4Hz, 1H), 3.47(d, J=17.4Hz, 1H), 2.17–2.09(m, 3H), 2.08...
Embodiment 66
[0071] Preparation of (S)-2-benzoyl-1-indanone-2-formic acid methyl ester IIIb-1 (phase transfer catalyst recycling)
[0072]
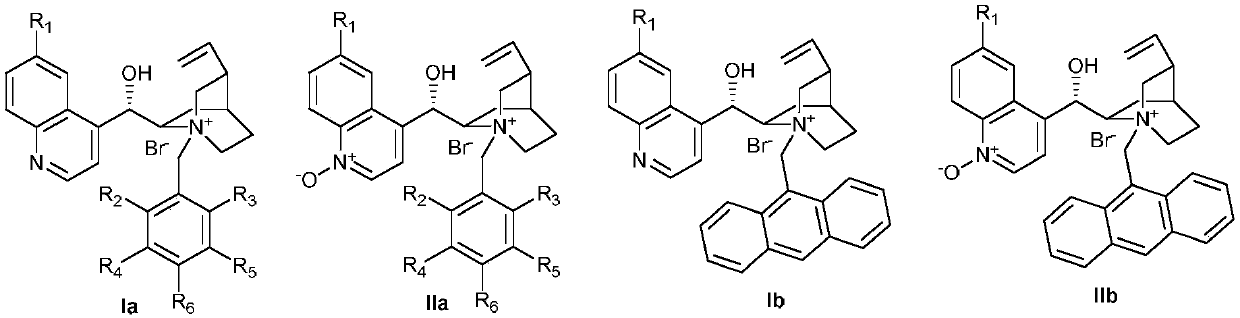
[0073] Weigh 2.5mmol 1-indanone-2-carboxylate adamantyl IIIa-1, add 2.5mol% phase transfer catalyst II-b, 3.8mmol benzoyl peroxide (mass fraction 70%), put into a 500mL reaction bottle, add K at a mass concentration of 30% 2 CO 3 Aqueous solution 25mL, 250mL toluene, stirred and reacted at 15 ℃, after reacting for 24 hours, the mixed liquid was separated into layers, the phase transfer catalyst was insoluble in the organic layer, suspended in the water layer, collected the organic layer and spin-dried the organic solvent, and separated by column chromatography ( Petroleum ether: ethyl acetate = 25:1) to obtain the asymmetric benzoylated product IIIb-1. Add 2.5mmol 1-indanone-2-carboxylate adamantyl IIIa-1, 3.8mmol benzoyl peroxide (mass fraction 70%), and 250mL toluene to the aqueous layer containing the phase transfer catalyst again, and continu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com