Preparation method of double-emission fluorescent probes for detecting content of aspirin in saliva
A dual-emission fluorescence and aspirin technology is applied in the field of preparation of dual-emission ratiometric fluorescent probes, which can solve the problems of complex sample processing, long time-consuming, and expensive detection costs, and achieve short process, stable structure and composition. Apply quick and easy effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
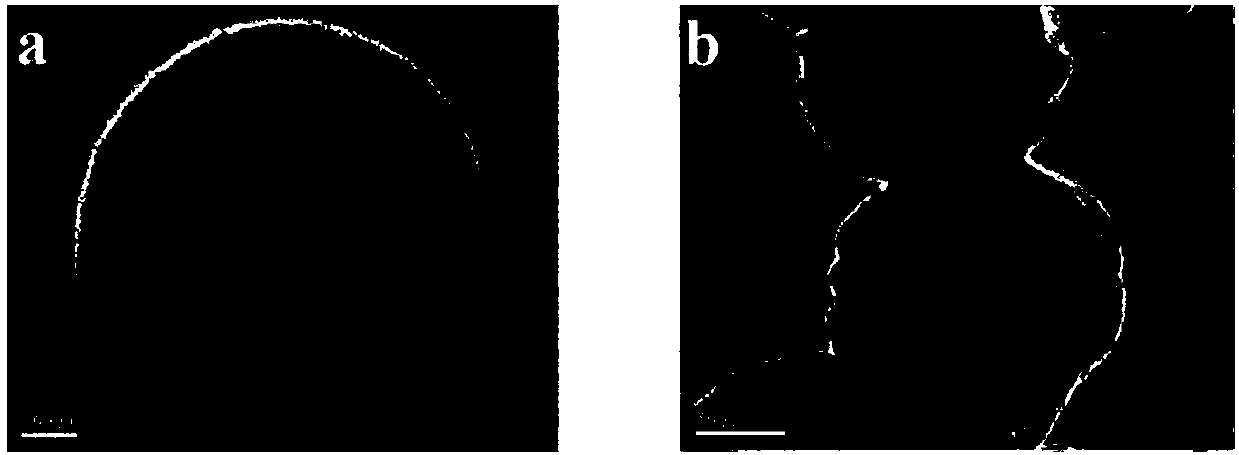
[0031] (1) Dissolve 0.5mmol tellurium powder in aqueous solution of sodium hydride tellurium, and add it to 1.0mmol cadmium chloride (CdCl 2 ) and 2.4 mmol thioglycolic acid (TGA) mixed solution, reflux at 100 °C for 2 hours and 24 hours, respectively, to prepare CdTe nanocrystals with green light emission (g-QDs) and red light emission (r-QDs).
[0032] (2) Mix 10 mL r-QDs solution and 40 mL ethanol solution in a conical flask, add 50 μL 3-aminopropyltriethoxysilane (APTES), and stir for 5 hours. Subsequently, 1.2 mL of tetraethoxysilane (TEOS) and ammonia water were added, stirring was continued for 12 hours, and 100 μL of 3-aminopropyltriethoxysilane (APTES) was added thereto. After the reaction was completed, it was centrifuged and washed several times to obtain r-QDs@SiO embedded in silicon coating. 2 Material.
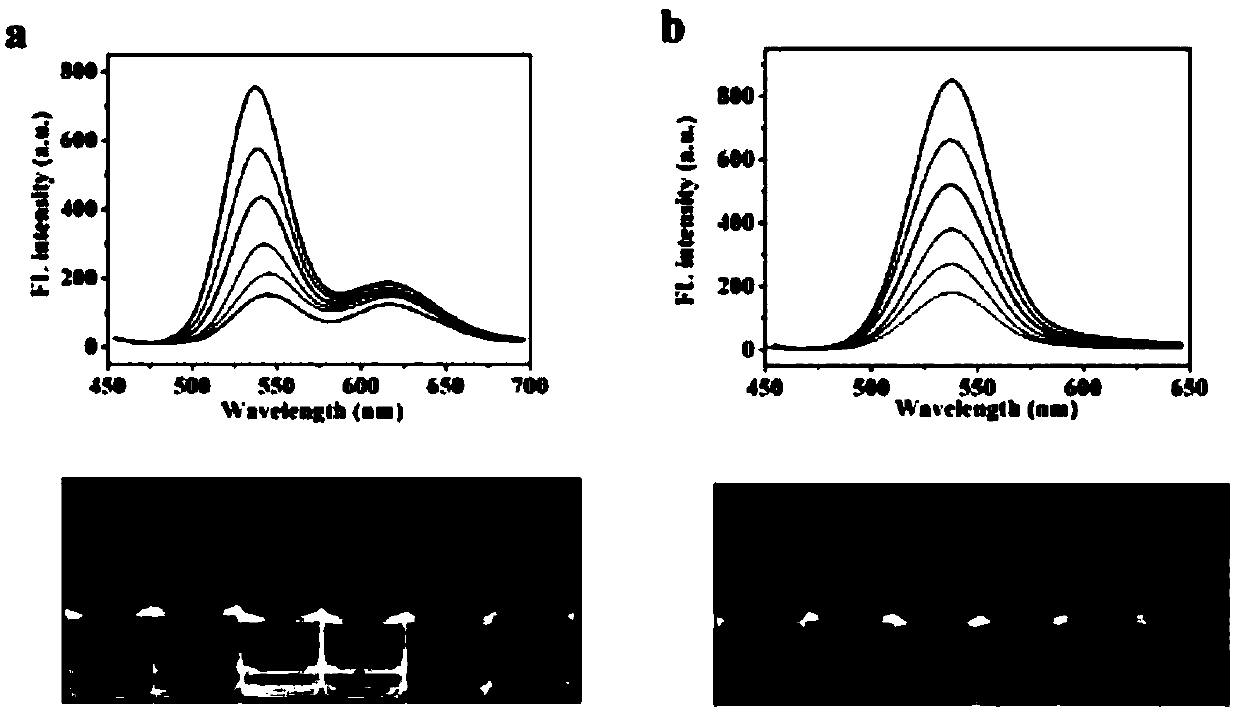
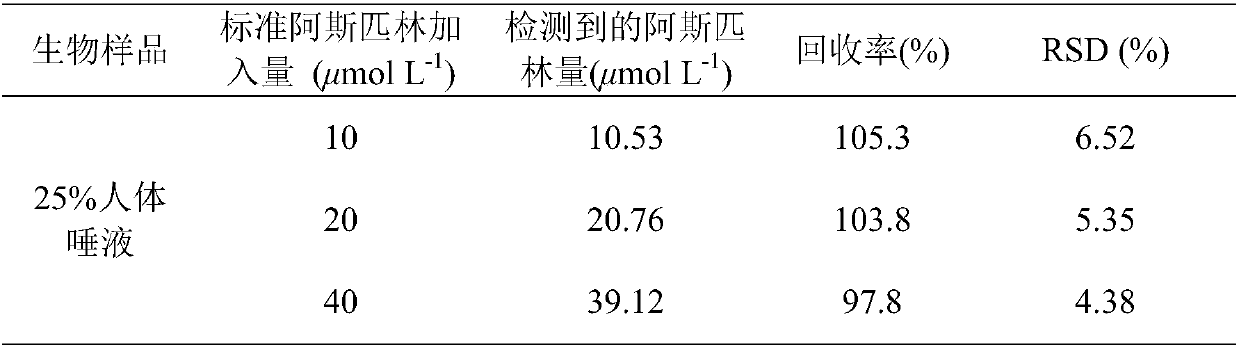
[0033] (3) Take 100μL r-QDs@SiO 2 Disperse with g-QDs in 12mg tris(hydroxymethyl)aminomethane buffer, then add 40μL 1-ethyl-3-(3-dimethylaminopropyl)carbodiim...
Embodiment 2
[0040] (1) Dissolve 0.5mmol tellurium powder in aqueous solution of sodium hydride tellurium, and add it to 1.0mmol cadmium chloride (CdCl 2 ) and 2.4mmol thioglycolic acid (TGA), reflux at 100°C for 3 hours and 22 hours, respectively, to prepare CdTe nanocrystals with green light emission (g-QDs) and red light emission (r-QDs).
[0041] (2) Mix 10 mL r-QDs solution and 40 mL ethanol solution in a conical flask, add 50 μL 3-aminopropyltriethoxysilane (APTES), and stir for 5 hours. Subsequently, 1.2 mL of tetraethoxysilane (TEOS) and ammonia water were added, stirring was continued for 12 hours, and 100 μL of 3-aminopropyltriethoxysilane (APTES) was added thereto. After the reaction was completed, it was centrifuged and washed several times to obtain r-QDs@SiO embedded in silicon coating. 2 Material.
[0042] (3) Take 100μL r-QDs@SiO 2 Disperse with g-QDs in 12mg tris(hydroxymethyl)aminomethane buffer, then add 40μL 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide and 36mg N-hy...
Embodiment 3
[0044] (1) Dissolve 0.5mmol tellurium powder in NaHTe aqueous solution, and add it to 1.0mmol cadmium chloride (CdCl 2 ) and 2.4mmol thioglycolic acid (TGA), reflux at 100°C for 4 hours and 20 hours, respectively, to prepare CdTe nanocrystals with green light emission (g-QDs) and red light emission (r-QDs).
[0045] (2) Mix 10 mL r-QDs solution and 40 mL ethanol solution in a conical flask, add 50 μL 3-aminopropyltriethoxysilane (APTES), and stir for 5 hours. Subsequently, 1.2 mL of tetraethoxysilane (TEOS) and ammonia water were added, stirring was continued for 12 hours, and 100 μL of 3-aminopropyltriethoxysilane (APTES) was added thereto. After the reaction was completed, it was centrifuged and washed several times to obtain r-QDs@SiO embedded in silicon coating. 2 Material.
[0046] (3) Take 100μL r-QDs@SiO 2 Disperse with g-QDs in 12mg tris(hydroxymethyl)aminomethane buffer, then add 40μL 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide and 36mg N-hydroxysuccinyl The mix...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com