Traditional Chinese veterinary medicine composition for treating swine enzootic pneumonia as well as preparation method and application thereof
A technology for swine asthma and a composition, applied in the field of traditional Chinese veterinary medicine, can solve problems such as lack of effective preparations and means, and achieve the effects of improving body immunity, reducing the number of administrations, and being less prone to recurrence.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
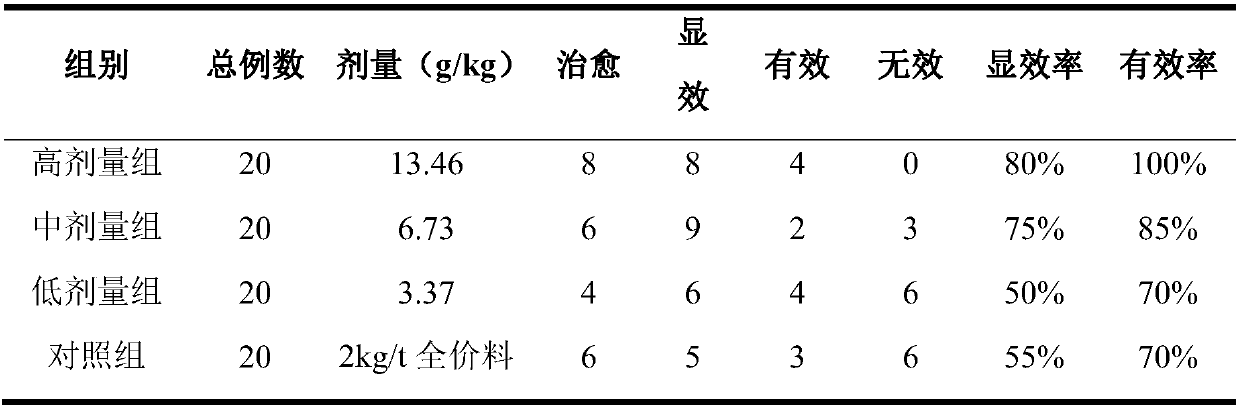
Examples
Embodiment 1
[0030] 1. Prescription composition:
[0031] 5kg of mulberry leaves, 20kg of chrysanthemums, 10kg of basil, 30kg of Houttuynia cordata, and 15kg of licorice.
[0032] 2. Preparation method:
[0033] (1) Take 20kg of chrysanthemums and 30kg of Houttuynia cordata, add water 8 times the weight of chrysanthemum and Houttuynia cordata, steam distill for 3 hours, collect volatile oil, dissolve with a small amount of ethanol, and obtain volatile oil ethanol solution, set aside; steam distillation The extract is filtered, and the filtrate and medicinal residues are used for subsequent use;
[0034] (2) Get 5 kg of mulberry leaves, 10 kg of mulberry leaves, and 15 kg of licorice, and after pulverization, add the dregs after extracting the volatile oil in step (1) to carry out water extraction, and then add 8 times the weight of mulberry leaves, radishes, and licorice. Water, reflux extraction twice, each time for 1.5 hours, the extract was filtered and concentrated; the concentrated ...
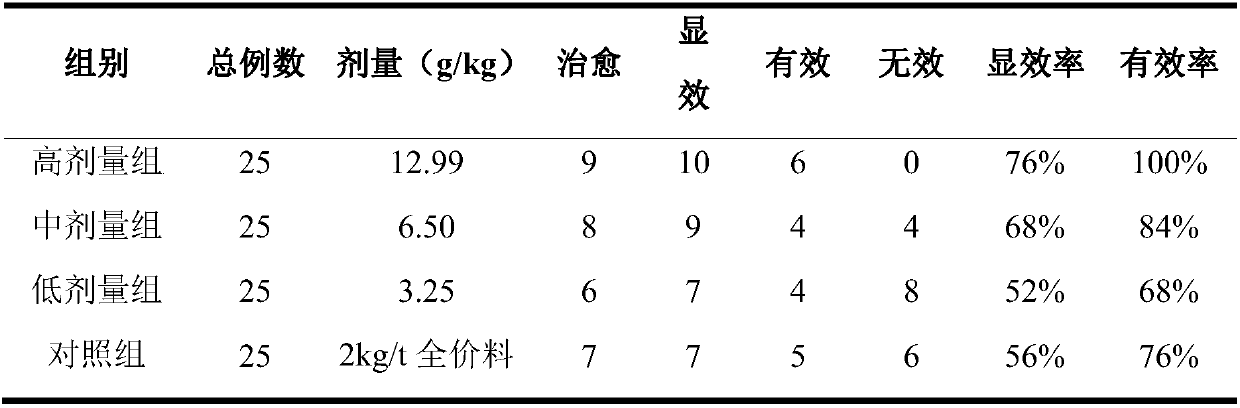
Embodiment 7
[0068] Embodiment 7: mouse acute toxicity test
[0069] 1. Purpose
[0070] Observe the acute toxic reaction of the Chinese veterinary drug composition prepared in Example 2 of the present invention to mice after oral administration, and evaluate the safety of the Chinese veterinary drug composition prepared in Example 2 of the present invention.
[0071] 2. Method
[0072] The maximum dose test method was used. 40 mice, weighing 25-30 g, half male and half male, were randomly divided into a control group and a drug group according to the preparation method of Example 2 of the present invention according to body weight, with 20 mice in each group. Example 2 of the present invention drug group with maximum concentration (4.30g crude drug / 1mL concentrated solution), maximum capacity (0.4mL / 10g body weight), gavage 2 times in 24h, 2 intervals 6 hours, observe animal behavior activities, mental state , skin color, stool and secretions, and body weight changes for 14 consecutive...
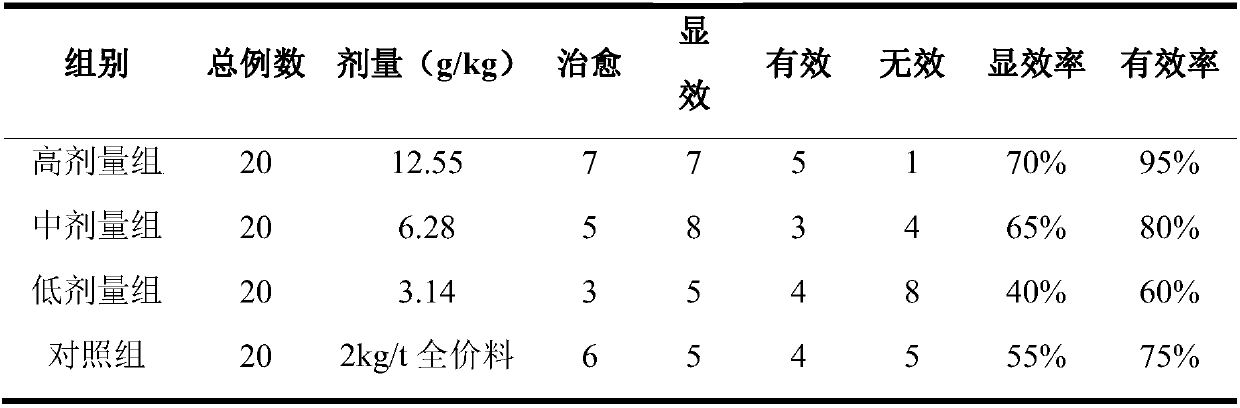
Embodiment 8
[0077] Embodiment 8: mouse acute toxicity test
[0078] 1. Purpose
[0079] Observe the acute toxic reaction of the Chinese veterinary drug composition prepared in Example 3 of the present invention to mice after oral administration, and evaluate the safety of the Chinese veterinary drug composition prepared in Example 3 of the present invention.
[0080] 2. Method
[0081] The maximum dose test method was used. 40 mice, with a body weight of 25-30 g, male and female, were randomly divided into a control group and a drug group according to the preparation method of Example 3 of the present invention, 20 in each group. Example 3 of the present invention drug group with maximum concentration (4.45g crude drug / 1mL concentrated solution), maximum capacity (0.4mL / 10g body weight), gavage 2 times in 24h, 2 intervals 6 hours, observe animal behavior activities, mental state , skin color, stool and secretions, and body weight changes for 14 consecutive days.
[0082] 3. Results
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com