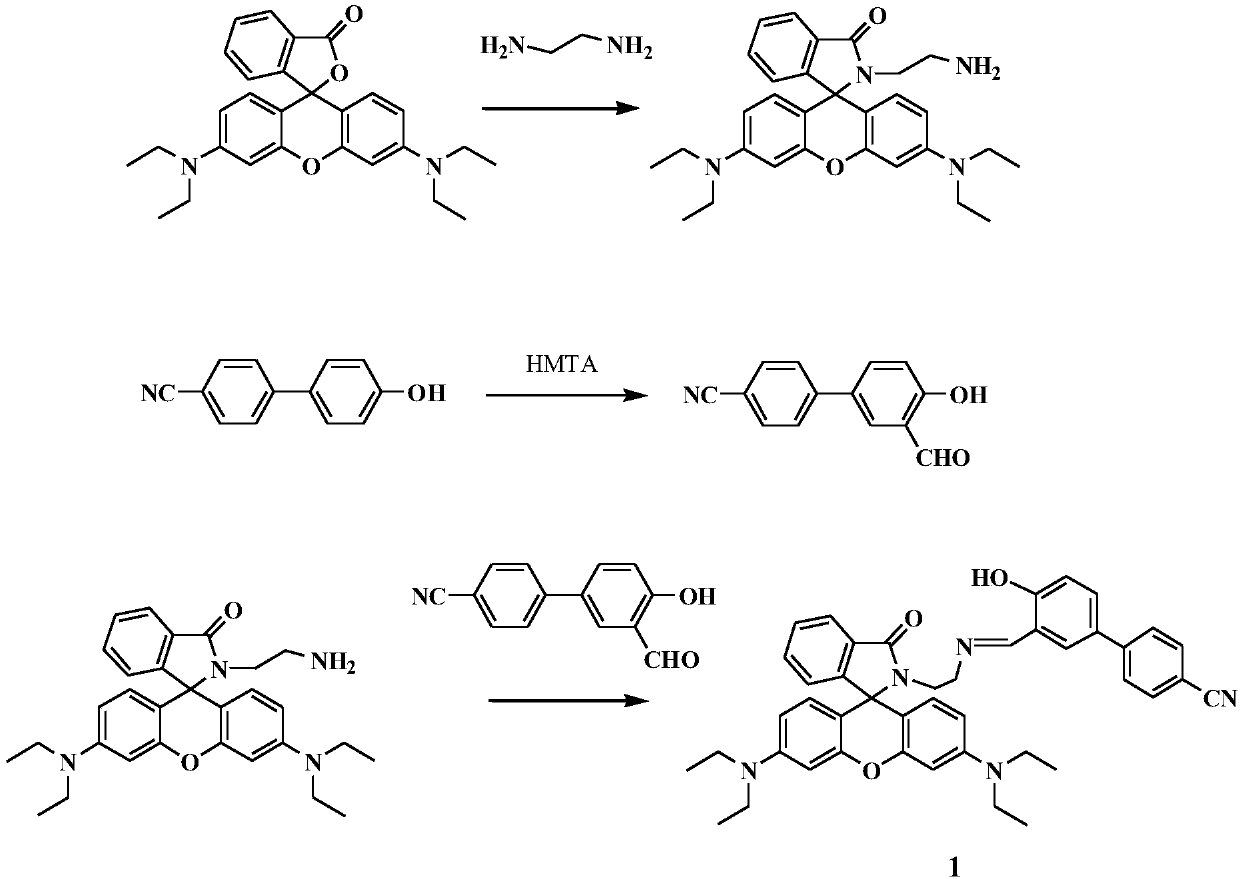
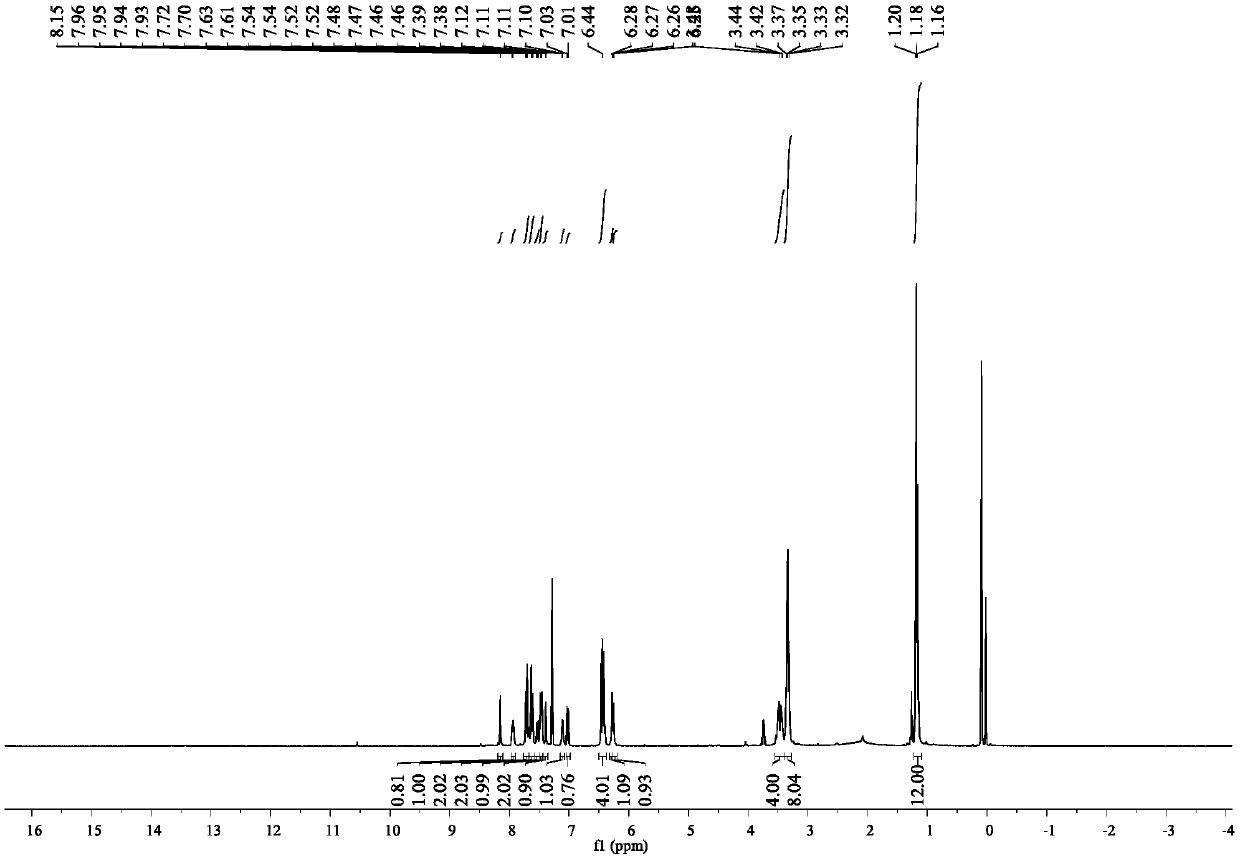
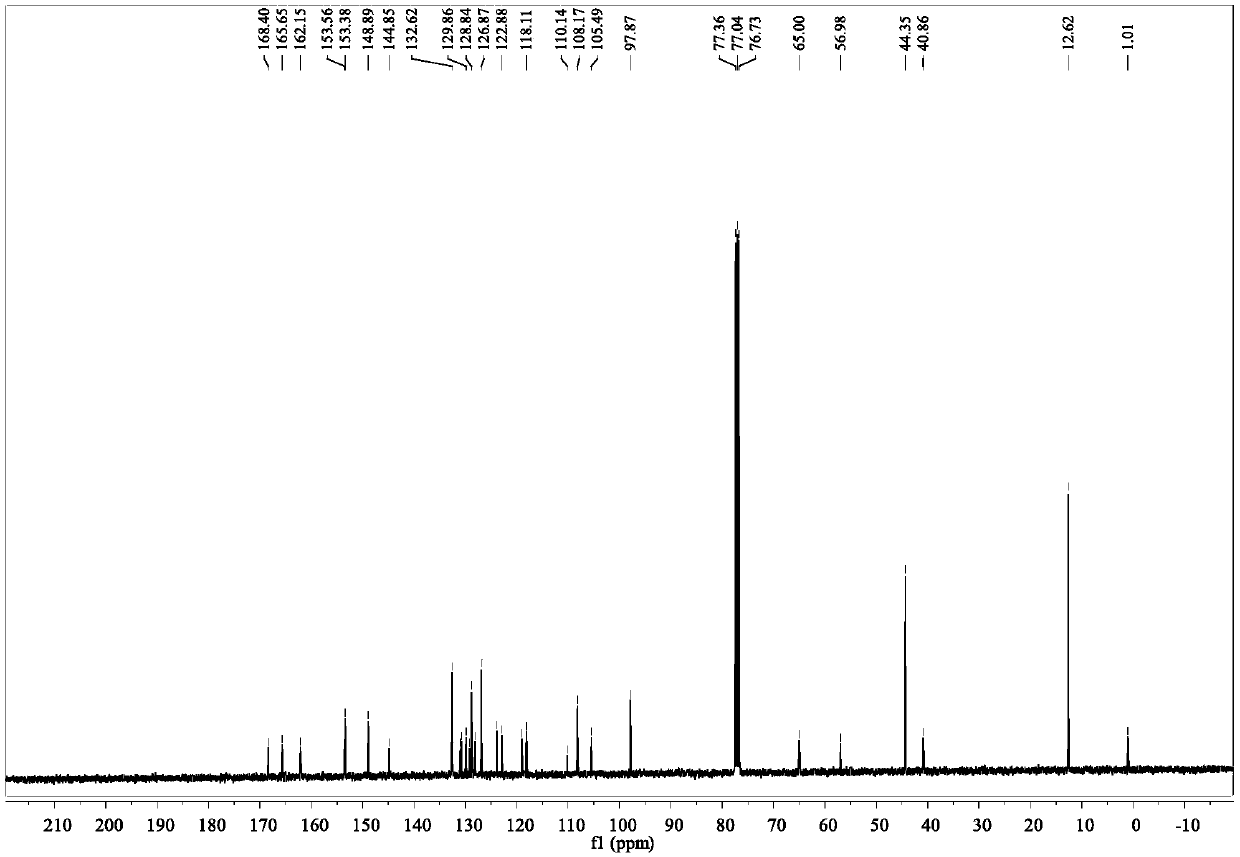
Fluorescence sensing material based on rhodamine B and hydroxy-4-biphenylcarbonitrile, and preparation and application thereof
A cyanobiphenol and fluorescent sensing technology, which is applied in the field of chemical fluorescent sensing materials, can solve the problems of expensive detection instruments, many interference factors of detection results, complex operation restrictions, etc., and achieve low preparation costs, good optical properties, The effect of multiple binding sites
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] S1. Synthesis of rhodamine ethylenediamine: put 1g (2mmol) rhodamine B in a 100mL round bottom flask, dissolve it completely with 20mL absolute ethanol, and add 0.18g (3mmol) ethylenediamine dropwise while stirring , placed in a 60°C oil bath and stirred at reflux for 8h. After the reaction was completed, it was cooled to room temperature, and the solvent was removed under reduced pressure to obtain a crude product of rhodamine amide, which was recrystallized and purified with absolute ethanol to obtain a pale pink product.
[0037] S2. Preparation of 3-formyl-4-hydroxy-4-biphenyl cyanide: Dissolve 0.39g (2mmol) 4-hydroxy-4-biphenyl cyanide and 1.41g (10mmol) urotropine in 30 mL In glacial acetic acid, place in a 100mL round-bottomed flask and stir to reflux in an oil bath at 90°C. After the reaction is finished, cool to room temperature, add 80 mL of 10M hydrochloric acid solution, stir at room temperature for acidification for 1 h, then extract with dichloromethane, ...
Embodiment 2
[0040] S1. Synthesis of rhodamine ethylenediamine: put 1.44g (3mmol) rhodamine B in a 100mL round bottom flask, dissolve it completely with 30mL of absolute ethanol, and add 0.27g (4.5mmol) of rhodamine B dropwise while stirring. Diamine, placed in a 65°C oil bath, stirred and refluxed for 10h. After the reaction was completed, it was cooled to room temperature, and the solvent was removed under reduced pressure to obtain a crude rhodamine diamide product, which was recrystallized and purified with absolute ethanol to obtain a pale pink product.
[0041] S2. Preparation of 3-formyl-4-hydroxy-4-biphenyl cyanide: Dissolve 0.59g (3mmol) 4-hydroxy-4-biphenyl cyanide and 2.11g (15mmol) urotropine in 50 mL In glacial acetic acid, place in a 100mL round-bottomed flask and stir to reflux in an oil bath at 100°C. After the reaction was completed, cool to room temperature, add 100mL of 10M hydrochloric acid solution and stir at room temperature for acidification for 1 h, then extract w...
Embodiment 3
[0044] S1. Synthesis of rhodamine ethylenediamine: put 2.4g (5mmol) Rhodamine B in a 100mL round bottom flask, dissolve it completely with 40mL of absolute ethanol, add 0.36g (6mmol) of Rhodamine B dropwise while stirring Diamine, placed in a 70°C oil bath, stirred and refluxed for 12h. After the reaction was completed, it was cooled to room temperature, and the solvent was removed under reduced pressure to obtain a crude rhodamine diamide product, which was recrystallized and purified with absolute ethanol to obtain a pale pink product.
[0045] S2. Preparation of 3-formyl-4-hydroxy-4-biphenyl cyanide: Dissolve 0.78g (4mmol) 4-hydroxy-4-biphenyl cyanide and 2.82g (20mmol) urotropine in 70 mL In glacial acetic acid, place in a 100mL round bottom flask and stir to reflux in an oil bath at 110°C. After the reaction is finished, cool to room temperature, add 120mL of 10M hydrochloric acid solution, stir at room temperature for acidification for 1 h, then extract with dichloromet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com