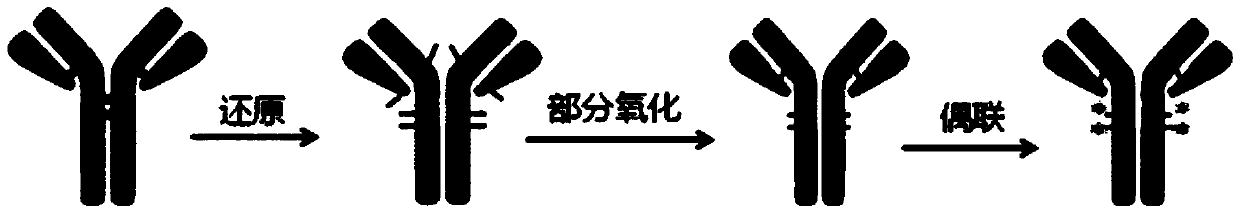
Bioconjugation method for IgG4 antibody
An antibody and conjugation technology, which is applied in the production of antibody-drug conjugates, can solve the problems of reduced utilization of antibody raw materials, increased separation and purification operations, and complicated production processes, so as to improve the utilization rate and simplify the production process , The effect of small requirements for reaction volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
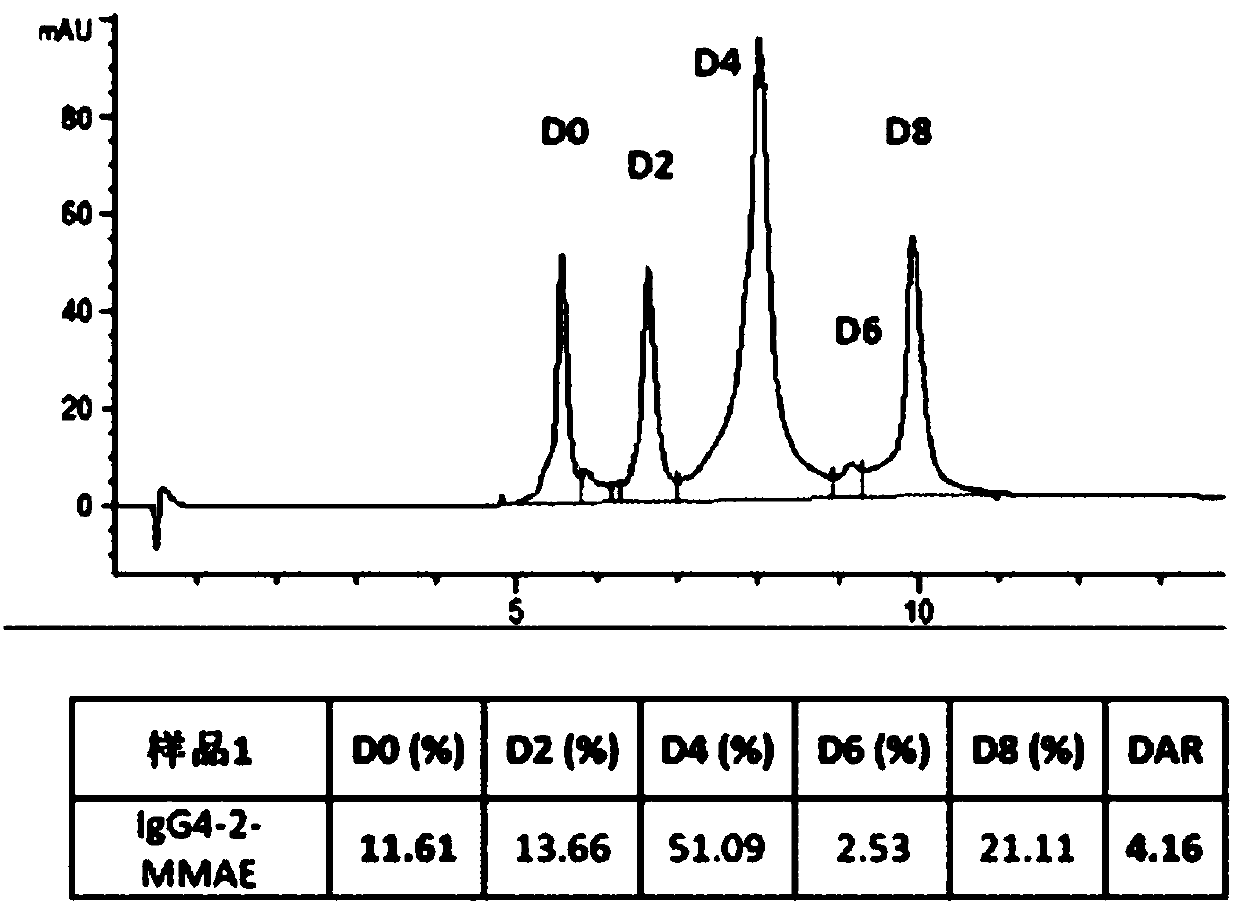
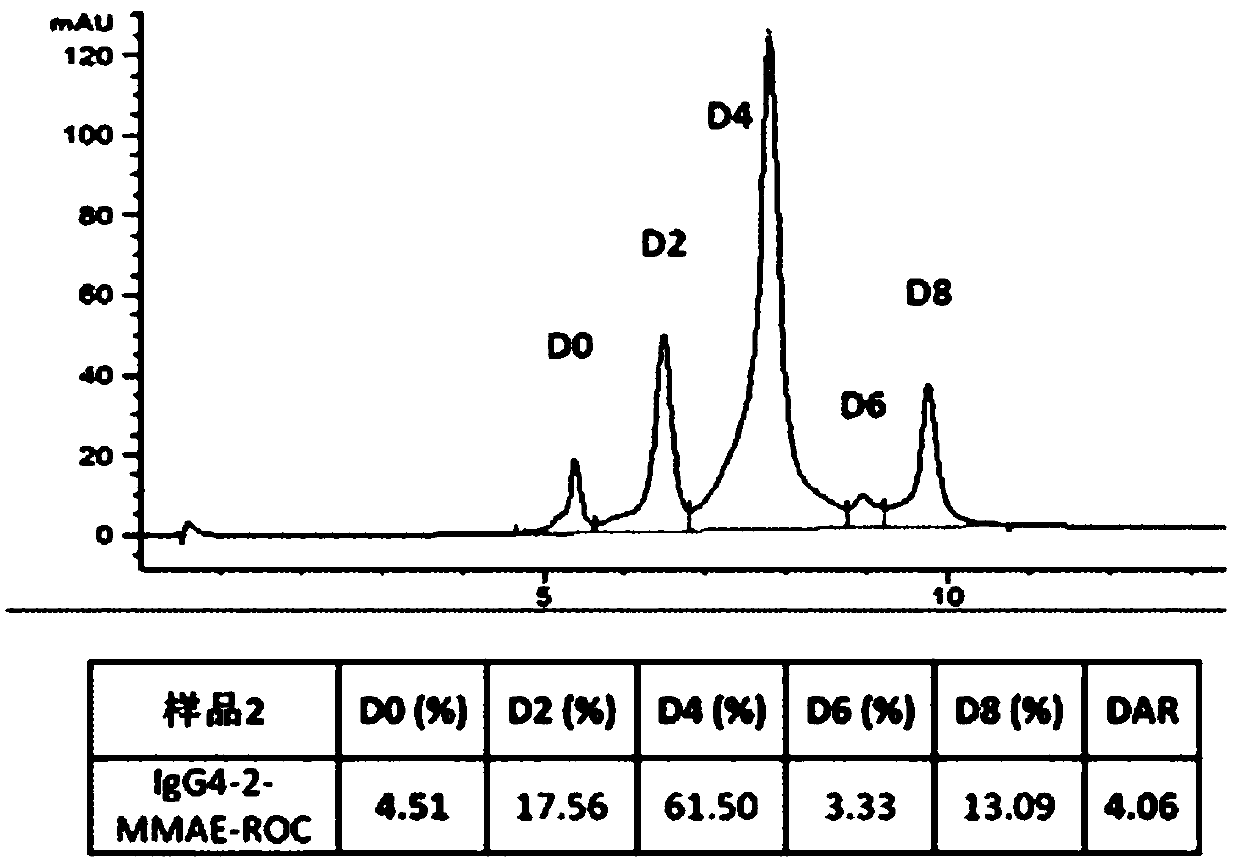
[0066] (1) Reduction of IgG4 antibody. Dissolve the antibody at a concentration of 8mg / ml in 20mM Histidine, 5% (w / v) sucrose, pH 5.5 buffer, add 20 equivalents of TCEP, place the reaction solution in a water bath at 37°C for 21 hours, PLRP -HPLC monitoring reaches the reaction end point (such as Figure 5 shown).
[0067] (2) Remove excess reducing agent. A desalting column with a molecular weight cut-off of 40KD was used to perform buffer exchange on the reduction reaction system. The antibody protein buffer was exchanged to 50mM NaPi, 50mM NaCl, 2mM EDTA, pH 6.5 while removing excess TCEP from the reaction.
[0068] (3) Partial oxidation of the reduced antibody. Add buffer, DMSO and DMSO stock solution of DHAA to the antibody purified by the desalting column, so that the antibody concentration is 5 mg / ml, the DMSO content is 5%, the equivalent ratio of DHAA to antibody is 6, and the reaction solution is placed at 4 ℃ for 3 hours, PLRP-HPLC monitoring reached the end of...
Embodiment 2
[0074] (1) Reduction of IgG4 antibody. Dissolve the antibody at a concentration of 10 mg / ml in PBS, pH 6.0 buffer, add 40 equivalents of DTT, place the reaction solution in a water bath at 37°C for 21 hours, and monitor by PLRP-HPLC to reach the end of the reaction (such as Figure 6 shown).
[0075] (2) Remove excess reducing agent. A desalting column with a molecular weight cut-off of 40KD was used to perform buffer exchange on the reduction reaction system. Buffer exchange the antibody protein to 50mM NaPi, 50mM NaCl, 2mM EDTA, pH 6.5 while removing excess DTT from the reaction.
[0076] (3) Partial oxidation of the reduced antibody. Add buffer, DMSO and DMSO stock solution of NAD to the antibody purified by the desalting column, so that the antibody concentration is 5 mg / ml, the DMSO content is 23%, and the equivalent ratio of NAD to antibody is 65. Place it at five degrees for 23 hours to complete the partial oxidation, so that the free sulfhydryl group reaches the ta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com