Preparation method of cobaltosic oxide with graded distribution of doping elements
A technology of cobalt tetroxide and doping elements, applied in the field of lithium ion batteries, can solve problems such as cumbersome reaction process, achieve the effect of environmental friendliness and avoid the impurity content exceeding the standard
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The production steps are the same as above, and the specific parameters in each step are as follows:
[0034] The prepared A solution is a cobalt chloride solution with a concentration of 60g / L and a volume of 120L.
[0035] The prepared B solution is 100g / L sodium hydroxide and 180g / L ammonia solution, and the volume ratio of sodium hydroxide to ammonia water is 1:20.
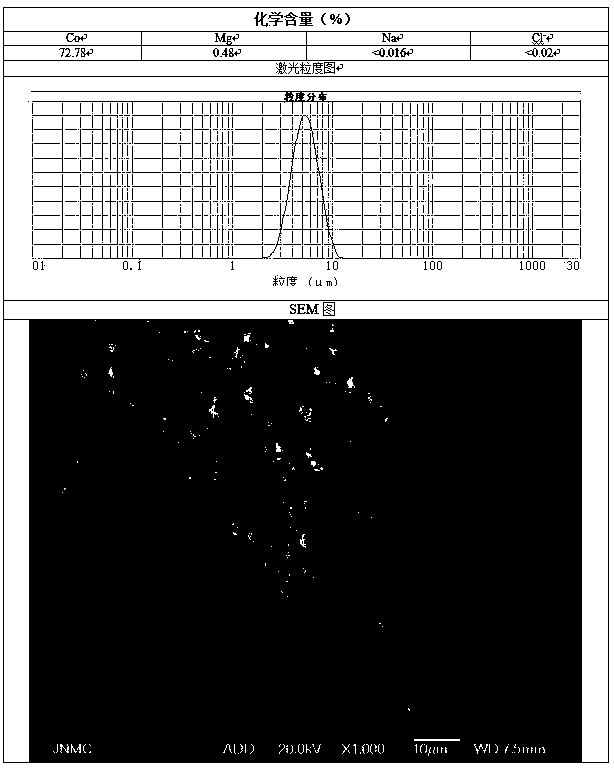
[0036] Preparation C solution is 10L of magnesium chloride solution of 5g / L, and the total amount of magnesium is about 0.5% of final product quality.
[0037] Prepare D solution as 1mol / L hydrogen peroxide solution.
[0038] At the beginning of the synthesis reaction, determine the reaction time according to the preparation process for 16 hours, add solution C to solution A at a flow rate of 0.625L / h, and simultaneously flow the mixed solution of A and C, solution B, and solution D at a flow rate of 8.125L / h Put it into the reaction kettle, and make the two react under strong stirring. The temperatur...
Embodiment 2
[0042] This example is basically the same as Example 1, except that the following adjustment parameters are different:
[0043] The prepared A solution is a cobalt nitrate solution with a concentration of 100g / L and a volume of 250L.
[0044] The prepared B solution is 200g / L sodium hydroxide and 180g / L ammonia solution, and the volume ratio of sodium hydroxide to ammonia water is 3:25.
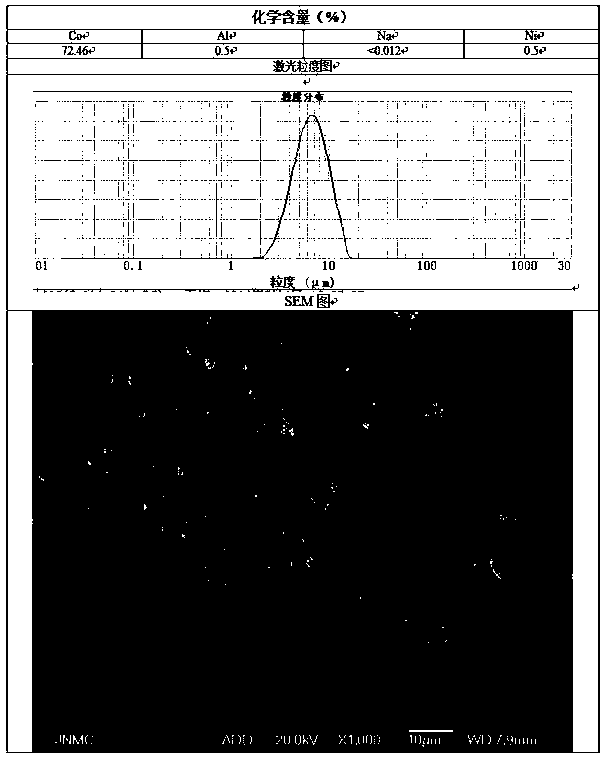
[0045] Prepare C solution as 10g / L aluminum and nickel solution 35L, and the total amount of Al and nickel is 1% of the final product mass.
[0046] Prepare the hydrogen peroxide solution whose D solution concentration is 2mol / L.
[0047] At the beginning of the synthesis reaction, determine the reaction time according to the preparation process for 30 hours, add solution C to solution A at a flow rate of 1.17L / h, and simultaneously flow the mixed solution of A and C, solution B, and solution D at a flow rate of 9.5L / h Put it into the reaction kettle, and make the two react under strong sti...
Embodiment 3
[0051] This example is basically the same as Example 1, except that the following adjustment parameters are different:
[0052] The prepared A solution is a cobalt sulfate solution with a concentration of 120g / L and a volume of 500L.
[0053]The prepared B solution is 300g / L sodium hydroxide and 180g / L ammonia solution, and the volume ratio of sodium hydroxide to ammonia water is 1:5.
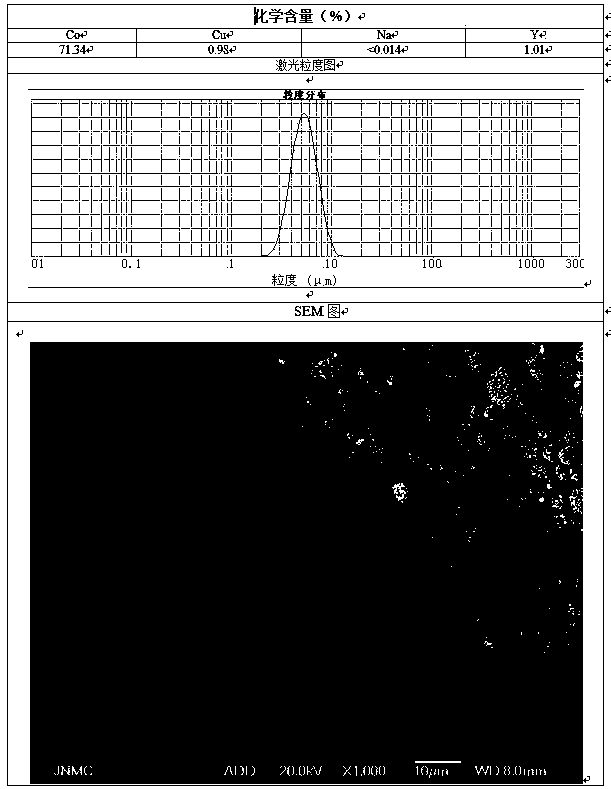
[0054] Preparation C solution is 84L of copper nitrate and yttrium chloride solution of 20g / L, and the total amount of copper and yttrium is about 2% of the final product quality.
[0055] Prepare the hydrogen peroxide solution whose D solution concentration is 4mol / L.
[0056] At the beginning of the synthesis reaction, according to the preparation process, the reaction time was determined to be 60h, and the C solution was added to the A solution at a flow rate of 1.4L / h. Flow into the reaction kettle, and make the two react under strong stirring. The temperature of the reaction system is 75...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com