Preparation method of gradient-doping lithium cobalt oxide
A technology of gradient doping and lithium cobalt oxide, applied in the field of lithium ion batteries, can solve the problem that doping ions cannot show gradient distribution, and achieve the effect of avoiding excessive impurity content and simple requirements.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The production steps are the same as above, and the specific parameters in each step are as follows:
[0038] The prepared solution A is a mixed solution of cobalt chloride and lithium chloride, and the cobalt concentration is 1mol / L, the lithium concentration is 1.2mol / L, and the volume is 120L.
[0039] The prepared B solution is 1.2mol / L ammonium bicarbonate solution.
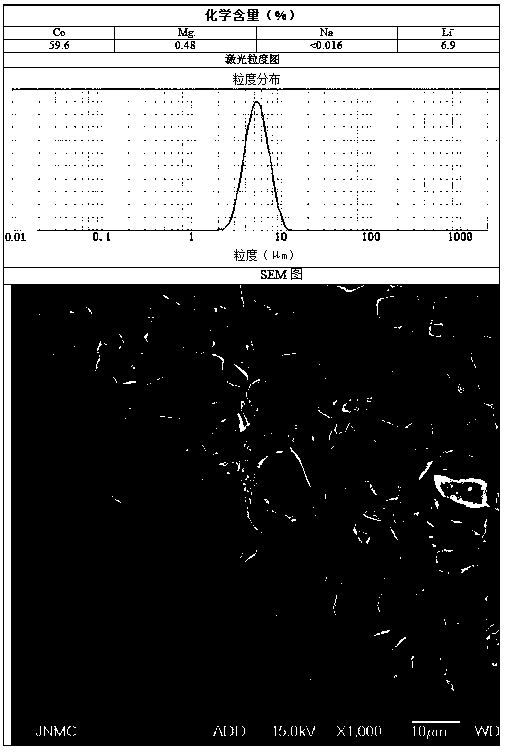
[0040] Preparation C solution is 10L of magnesium chloride solution of 5.9g / L, and the total amount of Mg is 0.5% of final product quality.
[0041] Prepare solution D as 2.5mol / L sodium hydroxide solution.
[0042] Prepare E solution as 2mol / L hydrogen peroxide solution.
[0043] At the beginning of the synthesis reaction, determine the reaction time according to the preparation process for 16 hours, add solution C to solution A at a flow rate of 0.625L / h, and simultaneously flow the mixed solution of A and C, solution B, and solution D at a flow rate of 8.125L / h Add it into a reaction kettle, and...
Embodiment 2
[0049] This example is basically the same as Example 1, except that the following adjustment parameters are different:
[0050] The prepared solution A is a mixed solution of cobalt nitrate and lithium nitrate, and the cobalt concentration is 1.5mol / L, the lithium concentration is 2mol / L, and the volume is 250L.
[0051] The prepared B solution is 2mol / L ammonium bicarbonate solution.
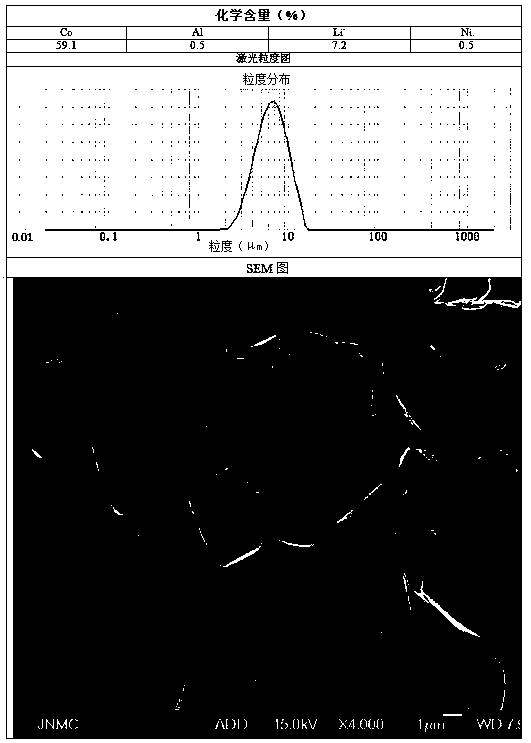
[0052]Preparation C solution is 12.3g / L aluminum nitrate and nickel chloride solution 30L, and the total amount of aluminum and nickel is 1% of the final product quality.
[0053] Prepare solution D as 5mol / L sodium hydroxide solution.
[0054] Prepare E solution as 3mol / L hydrogen peroxide solution.
[0055] At the beginning of the synthesis reaction, determine the reaction time according to the preparation process for 30 hours, add solution C to solution A at a flow rate of 1.0L / h, and simultaneously flow the mixed solution of A and C, solution B, and solution D at a flow rate of 9.3L / h Ad...
Embodiment 3
[0061] This example is basically the same as Example 1, except that the following adjustment parameters are different:
[0062] The prepared solution A is a mixed solution of cobalt sulfate and lithium chloride, and the cobalt concentration is 2mol / L, the lithium concentration is 2.5mol / L, and the volume is 500L.
[0063] The prepared B solution is 2.5mol / L ammonium bicarbonate solution.
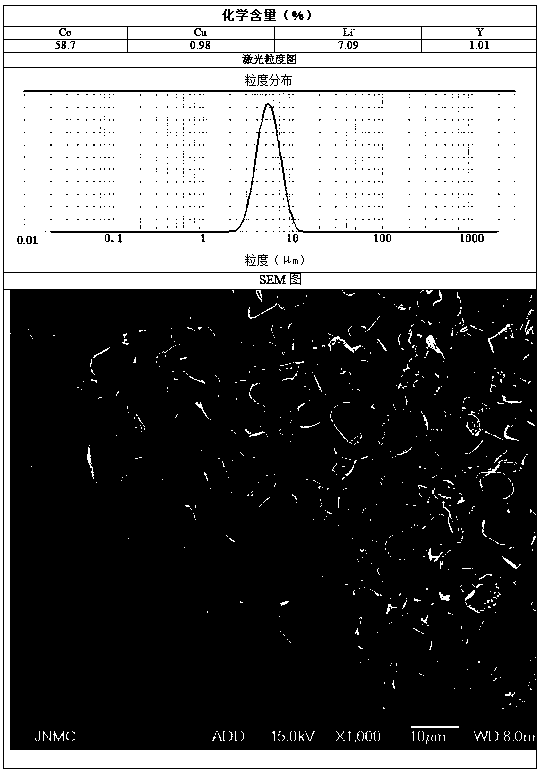
[0064] Preparation C solution is 100L of copper nitrate and yttrium chloride solution of 19.6g / L, and the total amount of copper and yttrium is 2% of the final product quality.
[0065] Prepare solution D as 4mol / L sodium hydroxide solution.
[0066] Prepare E solution as 4mol / L hydrogen peroxide solution.
[0067] At the beginning of the synthesis reaction, according to the preparation process, determine the reaction time of 40h, add the C solution to the A solution at a flow rate of 1.67L / h, and simultaneously flow the A, C mixed solution, B solution, and D solution at a flow rate of 10L...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com