Photoacid-generating compound, polymer derived therefrom, photoresist composition, and method of forming a photoresist relief image
A compound and photoacid technology, which is applied in the field of photoacid generation compounds, can solve the problems of uneven distribution of PAG compounds, reducing the solubility of PAG compounds, etc.
- Summary
- Abstract
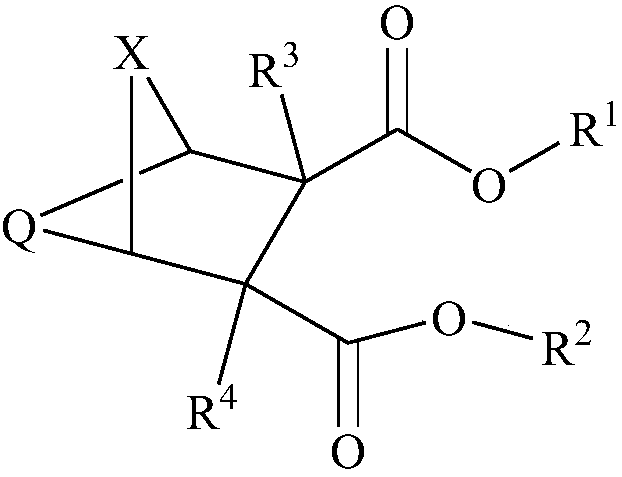
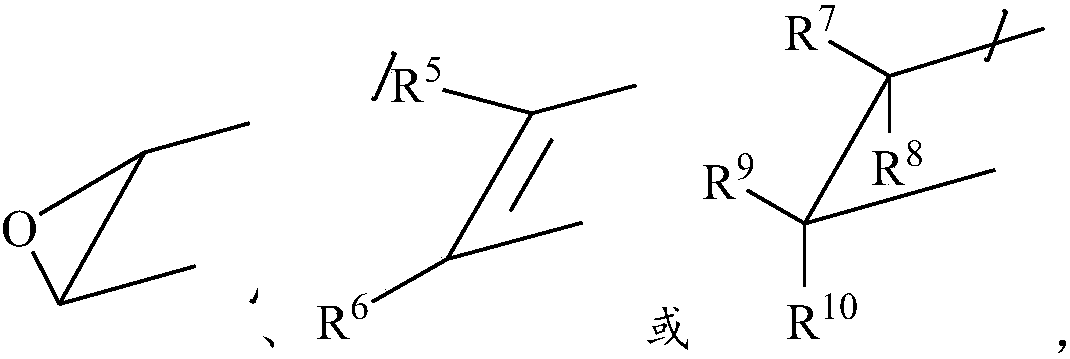
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
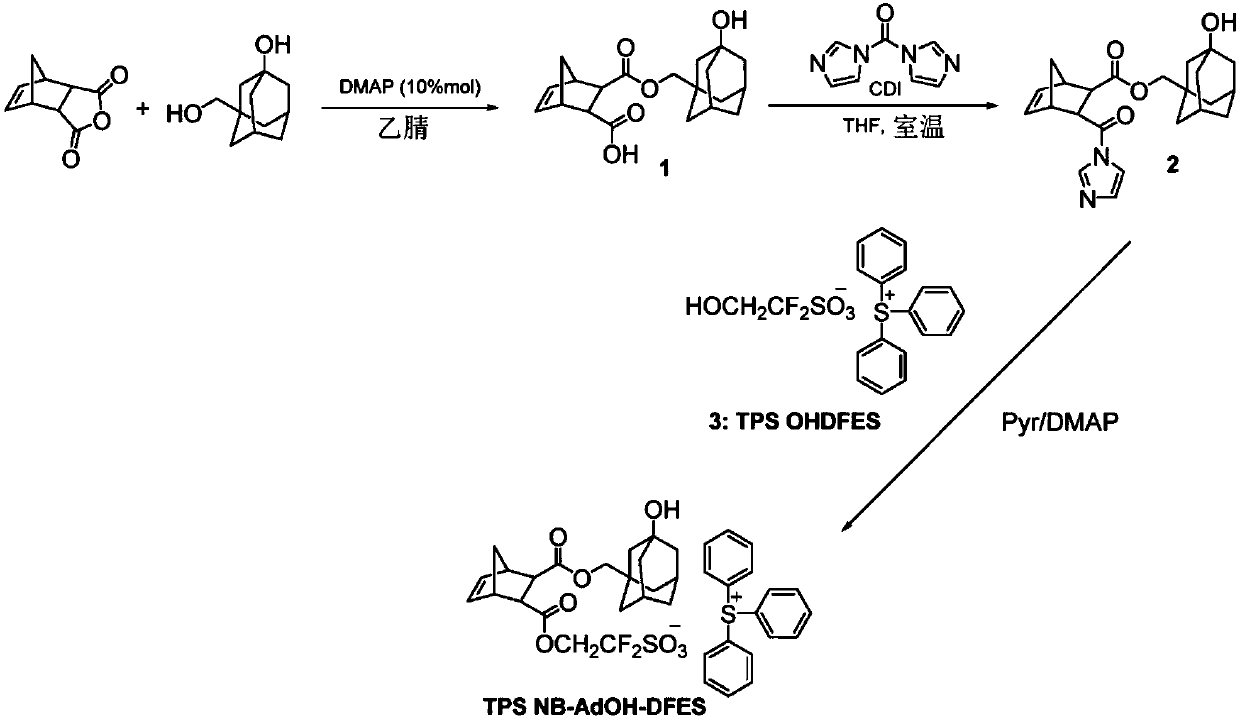
[0104] Example 1 - Synthesis of Photoacid Generator Compound TPS NB-AdOH-DFES
[0105] figure 1 The chemical scheme for the synthesis of a photoacid generator compound called TPS NB-AdOH-DFES is shown. The synthesis method is as follows. 5-norbornene-2,3-dicarboxylic anhydride (20 g, 122 mmol) and 3-(hydroxymethyl)adamantan-1-ol (22.2 g, 121.8 mmol) in acetonitrile (150 ml) N,N-Dimethylaminopyridine (1.5 g, 12.27 mmol) was added to the suspension in , and the reaction mixture was stirred at 65 °C for 18 h. The mixture was cooled to room temperature, and concentrated aqueous hydrochloric acid was added until the pH dropped to 2. The crude product was filtered and dried, then suspended in ethyl acetate (150 mL) and stirred at room temperature for 30 minutes. Filtration yielded 28 g (yield: 66%) of pure product (1) as a white solid. 1 H NMR (acetone-d6) δ: 6.22 (m, 1H), 6.17 (m, 1H), 3.69 (d, 2H), 3.53 (d, 2H), 3.40 (m, 2H), 3.14 (m, 2H), 2.17(s, 2H), 1.41-1.67(m, 15H).
...
example 2
[0107] Example 2 - Acid Diffusion Length Evaluation
[0108] The acid diffusion length is determined as follows. Polymer A1 (2-adamantyl-2-propyl methacrylate / α-(γ-butyrolactone) methacrylate / 1-hydroxyadamantyl-3-methacrylic acid Ester terpolymer, 30 / 50 / 20 molar ratio, Mw=10,000 g / mole) (5.981 weight percent of total formulation) and tert-butyl-4-hydroxypiperidine-1-carboxylic acid as quencher The ester (0.019 weight percent of the total formulation) was combined in a 50 / 50 (w / w) mixture of propylene glycol methyl ether acetate (PGMEA) and 2-hydroxyisobutyrate methyl ester (HBM) to prepare the acid assay Agent layer formulation.
[0109]
[0110] Polymer A1
[0111] Separately, by combining tert-butyl acrylate / methacrylic acid copolymer (70 / 30 molar ratio, respectively, 0.891% w / w solution) and photoacid generator compound PAG (inventive or comparative example) (153.40 micromol / grams, based on the total formulation) were combined in an 80 / 20 (w / w) mixture of 2-methyl-1...
example 4
[0136] Example 4 - Solubility Test
[0137] The solubility of photoacid generators was evaluated in a selection of organic solvents that could be used as formulation solvents or as negative tone photoresist developers. The solubility of each compound was obtained in an attempt to completely dissolve the compound at 2 weight percent at room temperature (23°C). The results of the solubility tests are shown in Table 4. Apparently, TPS from the present invention can be used in formulation solvents such as propylene glycol monomethyl ether acetate and propylene glycol monomethyl ether, and can be used as negative tone organic developers such as 2-ethanone and n-butyl acetate. The PAG of NB-AdOH-DFES has better solubility.
[0138] Table 4
[0139] Solvent / PAG
vs. PAG3
TPSNB-AdOH-DFES
Propylene glycol monomethyl ether acetate
X
O
O
O
Propylene Glycol Monomethyl Ether
X
O
Methyl 2-Hydroxyisobutyrate
O...
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
| distance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com