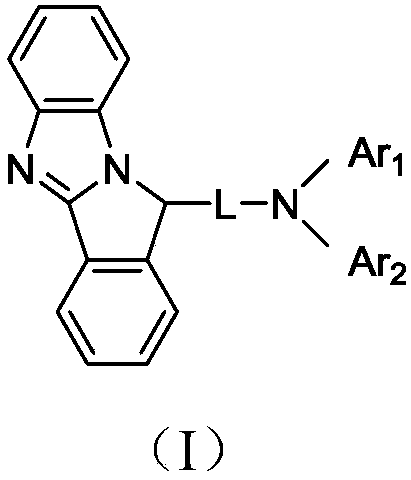
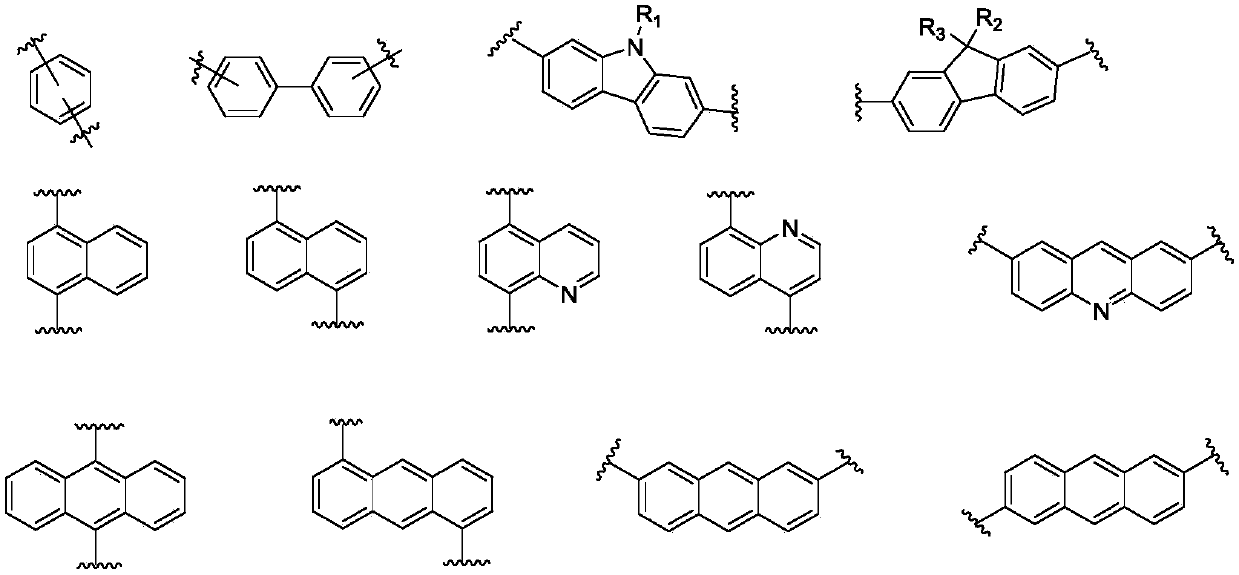
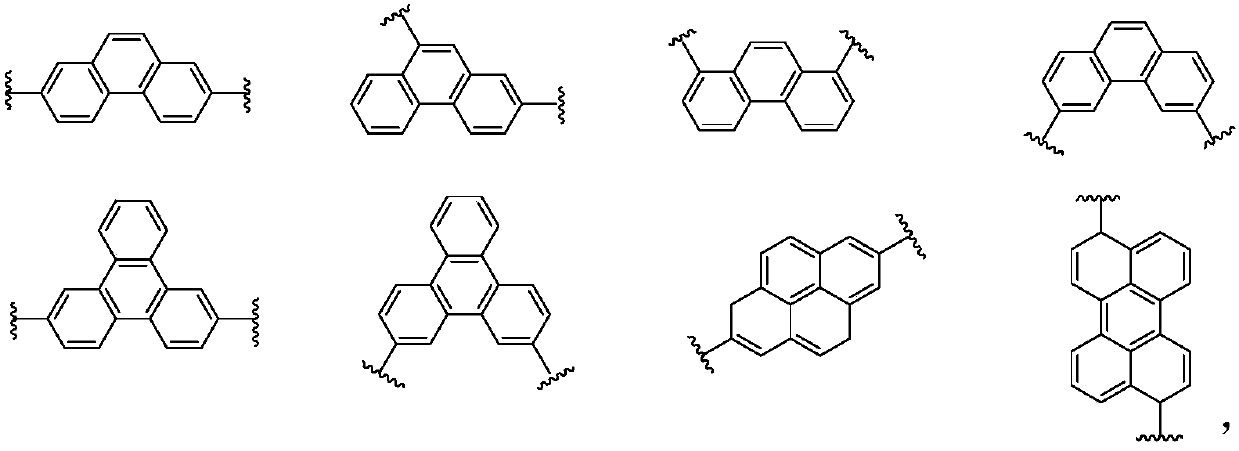
Imidazopyrrole derivative and organic light-emitting device using same
A technology of organic light-emitting devices and pyrrole derivatives, applied in light-emitting materials, organic chemistry, electro-solid devices, etc., can solve the problems of luminous efficiency and lifespan that hinder the commercialization of OLEDs, and achieve good industrialization prospects, high thermal stability performance, long life effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1: the synthesis of compound 1
[0060]
[0061] (1) Add compound I-1 (21.85g, 73.8mmol), compound II-1 (20.88g, 73.8mmol), t-BuONa (10.7g, 111mmol), Pd(OAc) in turn to the round bottom flask 2 (0.33g, 1.47mmol) and ultrasonically deoxygenated toluene (1.5L), refluxed under the protection of nitrogen, treated with ethyl acetate and water after the reaction solution was cooled, and the obtained organic layer was treated with MgSO 4 After drying, the solvent was evaporated under reduced pressure to obtain the crude product of compound III-1. With silica gel as the stationary phase and dichloromethane / hexane as the eluent, the crude product was subjected to column chromatography to obtain compound III-1 (40.58 g, 78 %).
[0062] (2) Compound III-1 (35.25g, 50mmol) was added into a round bottom flask, then an appropriate amount of anhydrous THF was added to dissolve it, and at a temperature of -78°C, n-BuLi( 4.9ml, 60mmol), after reacting for 0.5h, quickly d...
Embodiment 2
[0065] Embodiment 2: the synthesis of compound 5
[0066] According to the synthetic method of compound 1, compound 5 (9.93 g, 63%) was obtained.
[0067]
[0068] Mass Spectrum m / z: 525.23 (calculated: 525.22). Theoretical element content (%)C 38 h 27 N 3 : C, 86.83; H, 5.18; N, 7.99 Measured element content (%): C, 86.83; H, 5.19; N, 7.98. The above results confirmed that the obtained product was the target product.
Embodiment 3
[0069] Embodiment 3: the synthesis of compound 8
[0070] According to the synthetic method of compound 1, compound 8 (10.72 g, 65%) was obtained.
[0071]
[0072] Mass Spectrum m / z: 549.23 (calculated: 549.22). Theoretical element content (%)C 40 h 27 N 3 : C, 87.40; H, 4.95; N, 7.64 Measured element content (%): C, 87.41; H, 4.94; N, 7.64. The above results confirmed that the obtained product was the target product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com