Recombinant pichia pastoris for producing (+) gamma-lactamase as well as construction method and application thereof
A technology of lactamase and construction method, applied in the field of genetic engineering, can solve the problems of normal expression and less research on induced expression, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] In the present invention, the preparation method of the recombinant plasmid described in the above-mentioned technical scheme comprises the following steps:
[0032] A. The (+) γ-lactamase gene whose accession number is AE006641.1 in Genbank is amplified by the mutant primers T-L and T-R, and the PCR product is digested with restriction endonuclease Dpn Ⅰ for 2h; The mutant primer T-L has the nucleotide sequence shown in SEQ ID No.3 in the sequence listing, and the mutant primer T-R has the nucleotide sequence shown in SEQ ID No.4 in the sequence listing;
[0033] B. Connect the digested product to the T vector, transform E.coli DH5α, apply the transformed product to an LB plate containing 50 μg / ml kanamycin, and culture overnight at 37°C;
[0034] C. Extract the plasmid from the single colony picked, and use the primers γ-L and γ-R to amplify the target gene by PCR to obtain a PCR product; the γ-L has SEQ ID No.5 in the sequence table The nucleotide sequence shown, ...
Embodiment 1
[0074] Cloning and point mutation of γ-lactamase gene
[0075] Based on the thermostable γ-lactamase gene of Sulfolobus solfataricus with accession number AE006641.1 on NCBI, there is an endonuclease cleavage site in the gene according to the codon preference of Pichia pastoris Codon optimization is carried out, and the optimized codon is added when designing the primers.
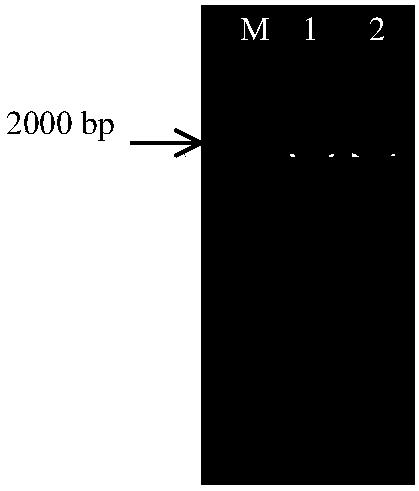
[0076] Specifically, the 591st base of the γ-lactamase gene was subjected to a point mutation, and the γ-lactamase gene was amplified using the mutation primers T-L and T-R, and the PCR product was digested with restriction endonuclease Dpn Ⅰ for 2 hours, and gelatinized with 1% agarose. Gel electrophoresis detection, the results are as follows figure 1 , where M represents 2K marker, 1 and 2 represent γ-lactamase gene
[0077] PCR reaction system:
[0078]
[0079] PCR reaction program: 95°C for 5min; 95°C for 30s, 62°C for 60s, 72°C for 10min, 18 cycles; 72°C for 20min; 16°C for 10min.
[0080] Dpn...
Embodiment 2
[0085] Recover the bands in the electrophoresis in Example 1, the target fragment is about 1500bp, connect it to the T carrier with T4 ligase, and transform E.coli DH5α, the specific steps are as follows: take the competent cell DH5α and place it in an ice bath, and place it in an ice bath. Add 5 μl of the recovered enzyme-cut product to 50 μl of competent cell suspension, flick and mix well, place in ice bath for 30 minutes, place the centrifuge tube in a 42°C water bath for 60-90 seconds, and quickly transfer the centrifuge tube to ice for 2- 3min, do not shake the centrifuge tube during this process. Add 800 μl of sterile LB medium (without antibiotics) to each centrifuge tube, mix well and place on a shaker at 37° C. for 45 minutes (180 rpm). Mix the contents of the centrifuge tube evenly, pipette 100 μl of transformed competent cells and add them to the LB plate containing 50 μg / ml kanamycin, spread evenly with a sterile spreader. Invert the plate and incubate overnight ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com