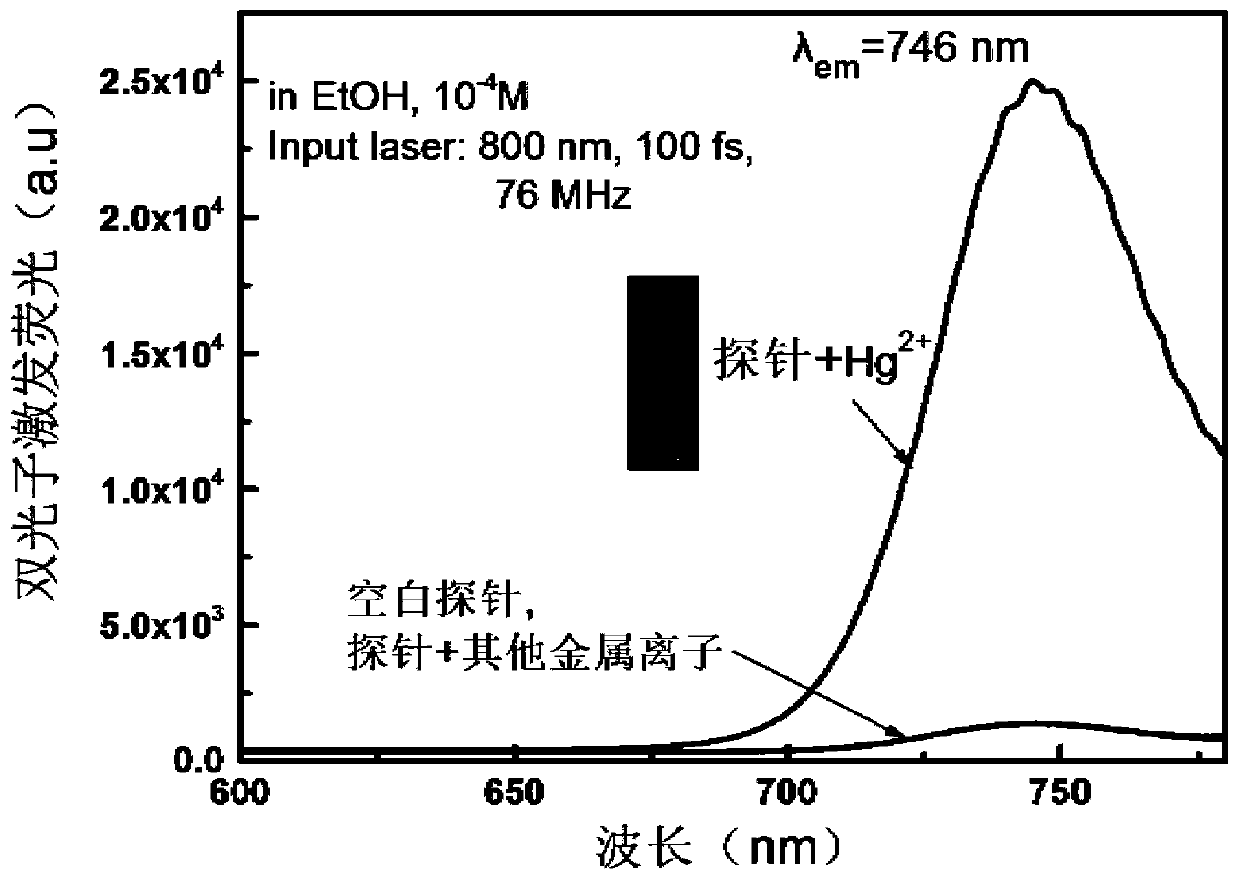
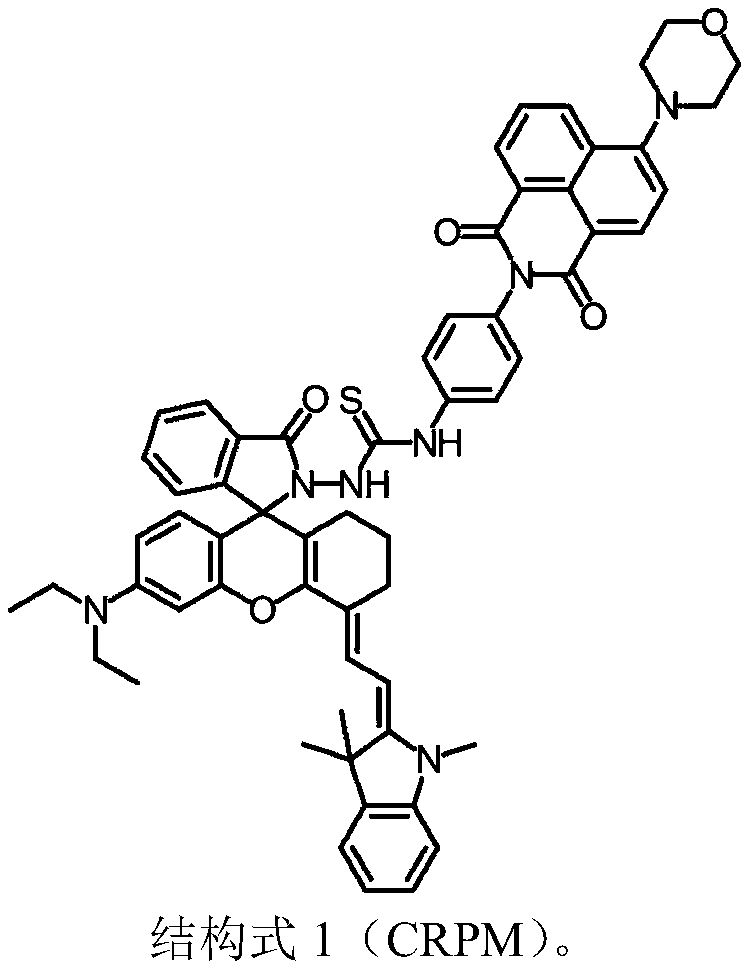
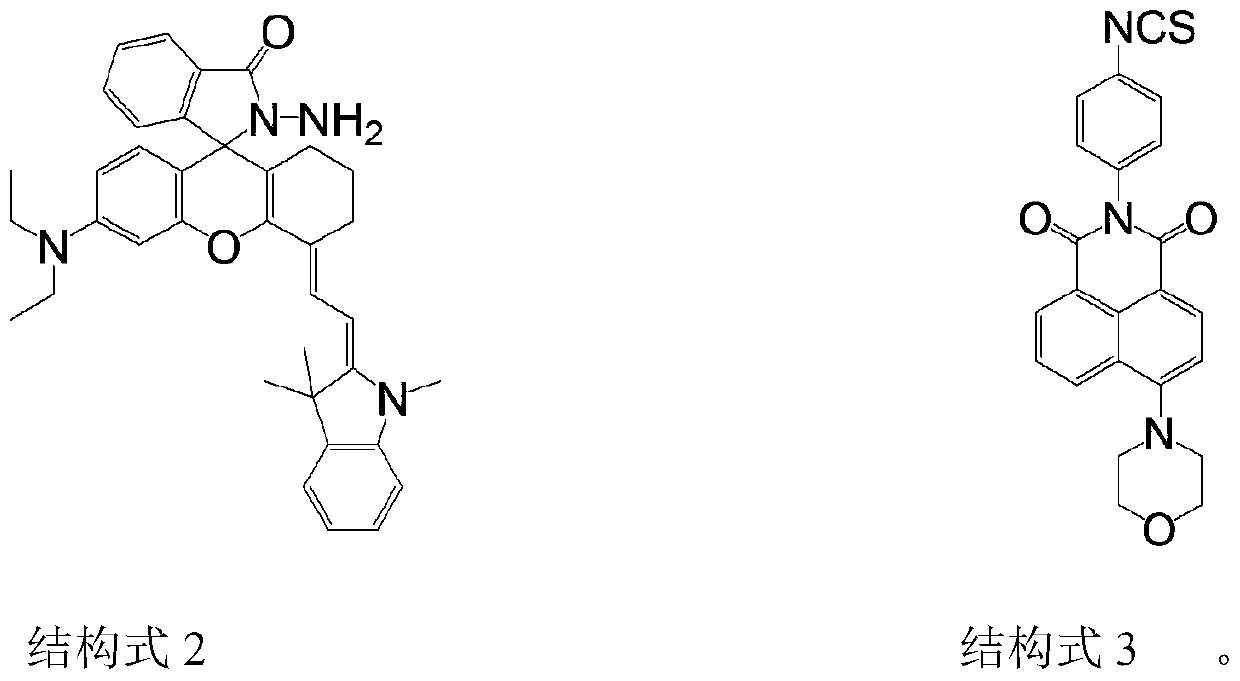
A near-infrared reactive two-photon fluorescent probe and its preparation method and application
A two-photon fluorescence and near-infrared technology, which is applied in the field of fluorescent probes, can solve the problems of limited application range and achieve high sensitivity, good biocompatibility, and wide application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] (1) The preparation method of fluorescent molecule CRPM is:
[0019] Under nitrogen protection, 1,3,3-trimethyl-2-methyleneindoline-rhodamine lactide (0.50 g, 0.87 mmol) was dissolved in 30 mL of anhydrous acetonitrile, and 4-morpholinyl was added. -1,8-Naphthalimide-phenyl isothiocyanate (0.36 g, 0.87 mmol), magnetically stirred, refluxed for 24 h, and the temperature was controlled at 80 °C. After the reaction was completed, CRPM was obtained by extraction, drying and purification by column chromatography with a yield of 45%.
[0020] The synthetic route of compound CRPM is as follows:
[0021]
[0022] Among them, 1,3,3-trimethyl-2-methyleneindoline-rhodamine hydrazide was prepared according to literature methods (JunyingXie, Chunyan Li, Yongfei Li, JunjieFei, Fen Xu, Juanou Yang, and Juan Liu.Near -InfraredFluorescent Probe with High Quantum Yield and Its Application in the Selective Detection of Glutathione in Living Cells and Tissues. Analytical Chemistry, 20...
Embodiment 2
[0030] The preparation method of fluorescent molecule CRPM is:
[0031] Under nitrogen protection, 1,3,3-trimethyl-2-methyleneindoline-rhodamine lactide (0.20 g, 0.35 mmol) was dissolved in 20 mL of anhydrous acetonitrile, and 4-morpholinyl was added. -1,8-Naphthalimide-phenylisothiocyanate (0.15 g, 0.35 mmol) was refluxed under magnetic stirring for 12 h, and the temperature was controlled at 80 °C. After the reaction was completed, CRPM was obtained by extraction, drying and purification by column chromatography with a yield of 40%.
Embodiment 3
[0033] The preparation method of fluorescent molecule CRPM is:
[0034] Under nitrogen protection, 1,3,3-trimethyl-2-methyleneindoline-rhodamine lactide (0.20 g, 0.35 mmol) was dissolved in 20 mL of anhydrous acetonitrile, and 4-morpholinyl was added. -1,8-Naphthalimide-phenylisothiocyanate (0.15 g, 0.35 mmol) was refluxed under magnetic stirring for 24 h, and the temperature was controlled at 75 °C. After the reaction was completed, CRPM was obtained by extraction, drying and purification by column chromatography with a yield of 45%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com