Preparation method of phenyl hydrazino thioformate-rhodamine fluorescent molecule, prepared fluorescent molecule and application of fluorescent molecule
A technology of hydrazine thiocarboxylate and chloromethyl thiophenyl ester is applied in the field of fluorescence sensors, which can solve problems such as selectivity influence, and achieve the effects of simple preparation method, good stability, good selectivity and sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Preparation of Phenylhydrazinothiocarbamate-Rhodamine Fluorescent Molecule CK
[0043] Rhodamine hydrazide was prepared according to the literature method (Chem. Commun., 2015, 51, 1697-1700). Under nitrogen protection, rhodamine B and hydrazine hydrate were refluxed in ethanol for 8 hours to obtain rhodamine hydrazide. The specific structural formula is:
[0044]
[0045] Phenylhydrazinothioformate-rhodamine fluorescent molecule CK is obtained by thioacylation reaction of rhodamine hydrazide and chloromethylthiophenyl ester. The specific synthesis method is as follows:
[0046] At room temperature, dissolve rhodamine hydrazide (456mg, 1.0mmol) in 10.0ml of dichloromethane, stir until the rhodamine hydrazide dissolves, then slowly add chloromethylthiophenyl ester (150μL, 1.1mmol) of dichloromethane solution, after the above mixed solution was reacted at room temperature for 16h, the organic solvent was removed under reduced pressure, and the crude product was separ...
Embodiment 2
[0055] Phenylhydrazinothiocarbamate-rhodamine fluorescent molecule CK on Sn 2+ Fluorescent response of
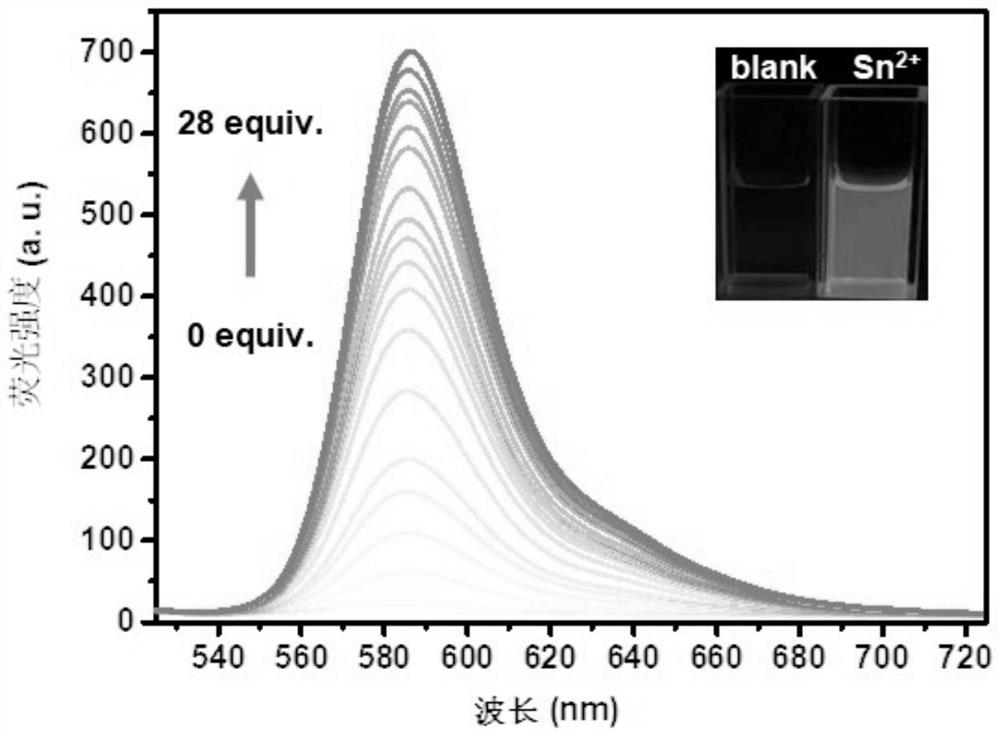
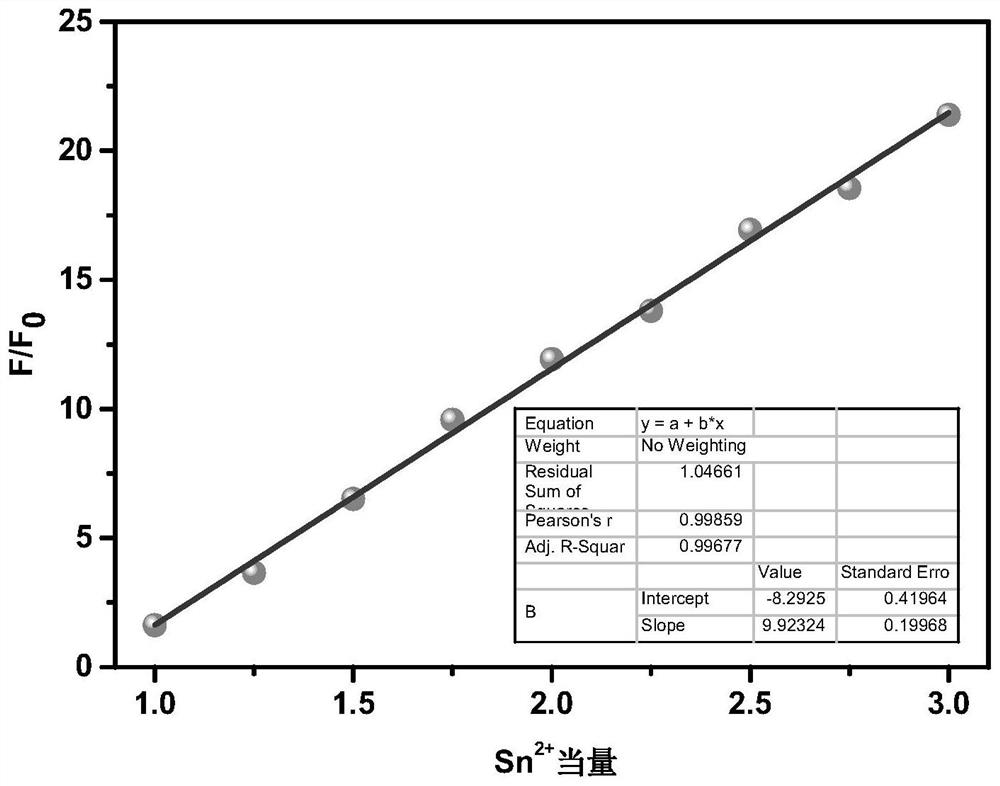
[0056] Method: Add the probe solution of phenylhydrazinothiocarbamate-rhodamine fluorescent molecule CK (water / ethanol=4:1, v / v) with a concentration of 10 μM into the cuvette, and scan the fluorescence spectrum , and then dropwise added 0-28 equivalents of Sn to the probe solution 2+ After the solution was thoroughly shaken and mixed at room temperature, the fluorescence spectrum scanning was carried out respectively. Among them, the fluorescence excitation wavelength is set to 500nm, and the slit: 10 / 10nm.
[0057] the result shows: figure 1 and figure 2 Add different concentrations of Sn to the hydrazinothiocarbamate-rhodamine fluorescent molecule CK solution 2+ Fluorescence spectra and linear relationship diagrams. Such as figure 1 and figure 2 As shown, the phenylhydrazinothiocarbamate-rhodamine fluorescent molecule CK itself has almost no fluorescence, with ...
Embodiment 3
[0059] Phenylhydrazinothiocarbamate-rhodamine fluorescent molecule CK on Sn 2+ response time
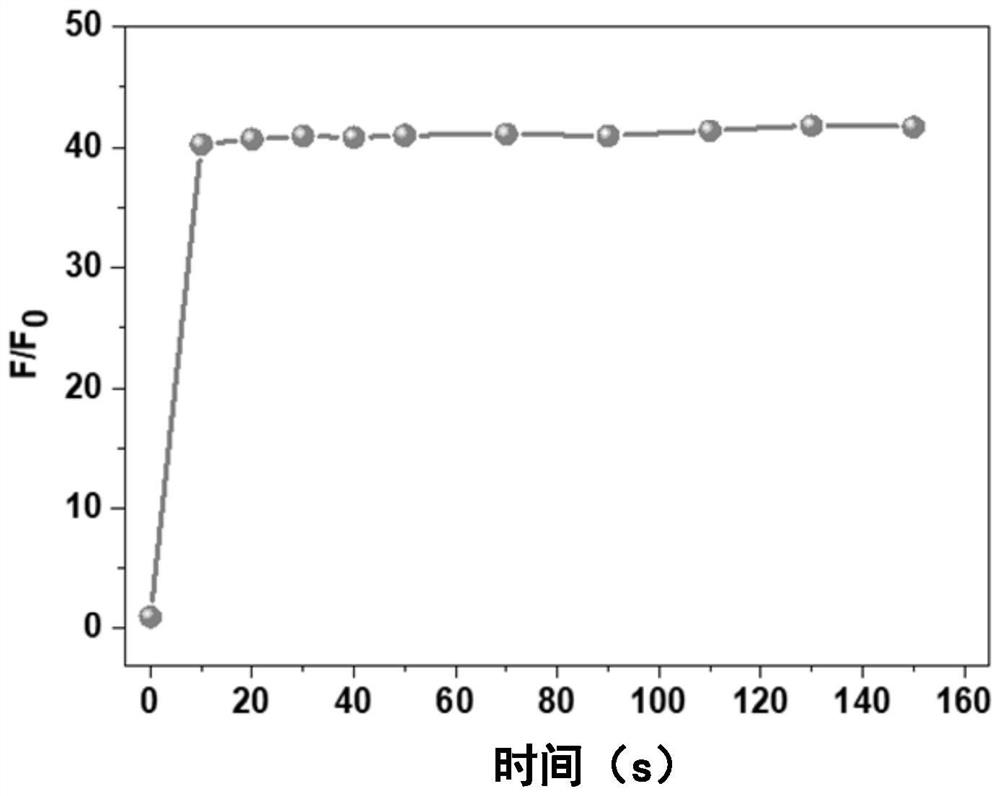
[0060] Method: Take the probe solution of phenylhydrazinothiocarbamate-rhodamine fluorescent molecule CK (water / ethanol=4:1, v / v) with a concentration of 10 μM and 20 equivalents of Sn 2+ The solution is added into a cuvette, detected on a fluorescence spectrophotometer, and scanned at intervals until the fluorescence intensity is basically stable. Among them, the fluorescence excitation wavelength is set to 500nm, and the slit: 10 / 10nm.
[0061] the result shows: image 3 Phenylhydrazinothiocarbamate-rhodamine fluorescent molecule CK in water / ethanol (volume ratio 4:1) and Sn 2+ Time-dependent fluorescence spectra of the reactions. Such as image 3 As shown, the effect of phenylhydrazinothiocarbamate-rhodamine fluorescent molecule CK on Sn 2+ The response speed is very fast, and the response reaches a balance within 20s and completes the Sn 2+ detection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com