Method for separating and determining pasiniazide and related impurities thereof by HPLC method
A technology for pasoniazid and related substances, applied in the field of analytical chemistry, can solve problems such as control, and achieve the effects of ensuring quality controllable, precise quality controllability, and improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Chromatographic conditions:
[0068] Chromatographic column: Shimadzu VP ODS 250×4.6mm, 5.0μm
[0069] Column flow rate: 1.0ml / min
[0070] Detection wavelength: UV 280nm
[0071] Column temperature: 30°C
[0072] Injection volume: 20μl
[0073] Solvent: buffer salt (disodium hydrogen phosphate 8.7g, sodium dihydrogen phosphate 3.4g, add water to dissolve and dilute to 1000ml)-methanol=90:10
[0074] Mobile phase: A: Phosphate buffer (8.7g disodium hydrogen phosphate, 3.4g sodium dihydrogen phosphate, 2.0g tetrabutylammonium hydrogen sulfate, add water to dissolve, and dilute to 1000ml)
[0075] B: Methanol
[0076] Gradient elution:
[0077]
[0078]
[0079] 1. Solution preparation
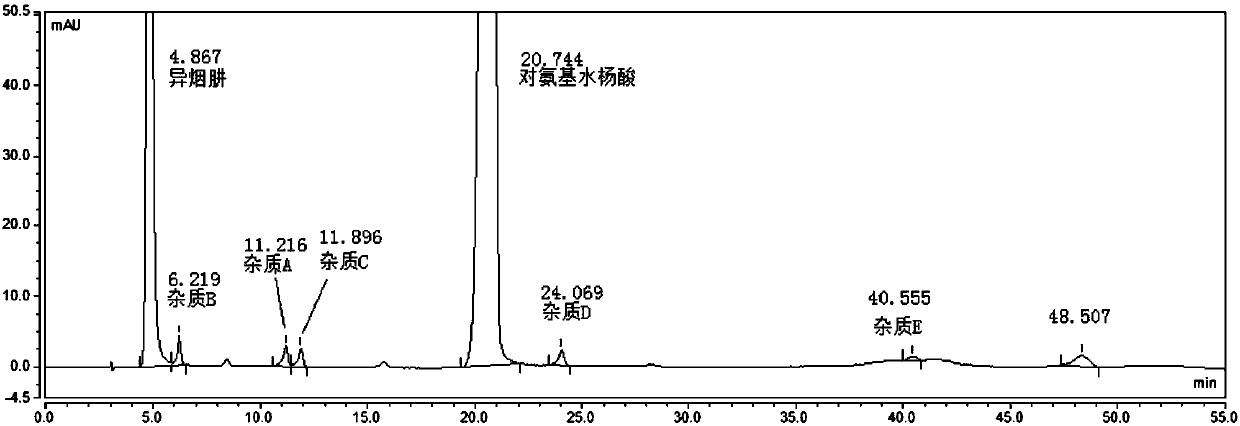
[0080] System suitability solution: take the appropriate amount of pasniazid reference substance, impurity A, impurity B, impurity C, impurity D, and impurity E, dissolve in methanol and dilute with a solvent to make about 1 mg of pasniazid and impurity per 1 ml 2ug mixed so...
Embodiment 2
[0087] Embodiment 2 comparative example
[0088] The national drug standard [WS-10001-(HD-0322)-2002] contains the quality standard of this product, and the relevant substances are not controlled;
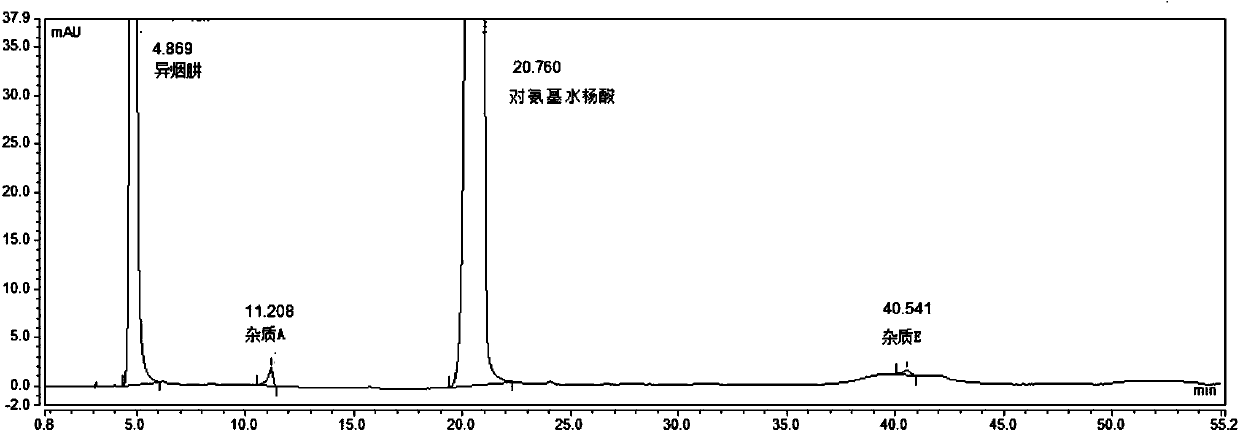
[0089] According to the Chinese Pharmacopoeia 2015 edition persinazid related substance items only m-aminophenol (impurity B) is controlled, using isocratic elution, the mobile phase is: phosphate buffer (disodium hydrogen phosphate 8.7g, sodium dihydrogen phosphate 3.4g, add appropriate amount of water to dissolve, 10% tetrabutylammonium hydroxide solution 23ml, and dilute to 1000ml)-methanol=90:10. As a result, it was found that the main peak of impurity B and isoniazid could not be effectively separated under the conditions of this chromatographic system; the main peak of impurity D (a possible degradation impurity) coincided with the main peak of p-aminosalicylic acid and could not be effectively detected; the peak of impurity E appeared under isocratic elution conditions The ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com