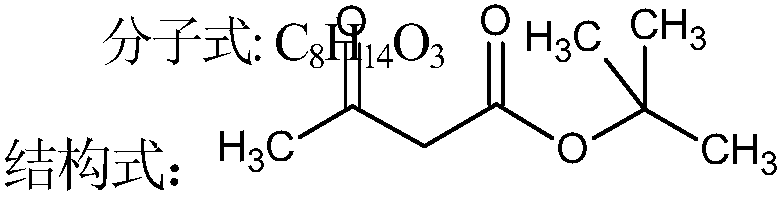
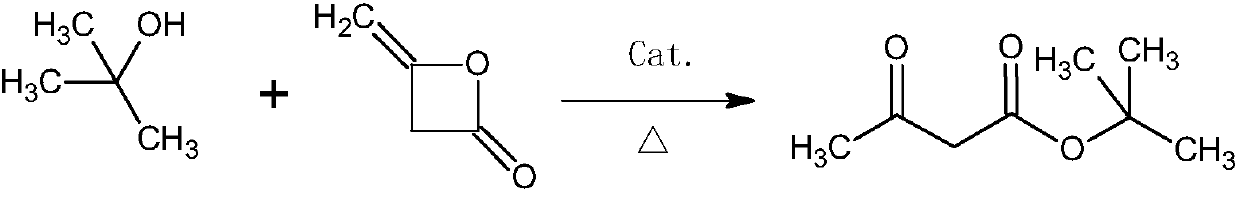Synthesis method of tert-butyl acetoacetate
A technology of tert-butyl acetoacetate and synthesis method, which is applied in the preparation of ketene/polyketene, organic chemistry, etc., can solve the problems of expensive raw materials, immature industrialization reports, and high price, and achieve an increase in reaction rate and selectivity, reducing steric hindrance effects, and improving reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] In a 1000ml flask equipped with stirring, a thermometer and a reflux condenser, add 370g (5.0mol) of tert-butanol and 0.79g (0.1%) of triethylenediamine, and add 420g (5.0mol) of diketene dropwise at room temperature, the reaction process The middle reaction temperature was controlled at 100° C., the dropwise addition was completed, and the temperature was controlled at 110° C. for 4 hours to finish the reaction. The crude ester was rectified under negative pressure to obtain 744 g of finished tert-butyl acetoacetate, with a yield of 94.1% and a content of 99.0%.
Embodiment 2
[0042] In a 1000ml flask equipped with stirring, a thermometer and a reflux condenser, add 518g (7.0mol) of tert-butanol and 2.81g (0.3%) of triethylamine, raise the temperature to 80°C, and dropwise add 420g (5.0mol) of diketene During the reaction, the reaction temperature was controlled at 90° C., and the dropwise addition was completed. The temperature was controlled at 90° C. and kept for 8 hours to end the reaction. The crude ester was rectified under negative pressure to obtain 745 g of finished tert-butyl acetoacetate, with a yield of 94.3% and a content of 99.3%.
Embodiment 3
[0044] In a 1000ml flask equipped with stirring, a thermometer and a reflux condenser, add 444g (6.0mol) of tert-butyl alcohol, 9g (2%) of triethylenediamine and 8.28g of triethylamine, and heat up to 50°C. 420g (5.0mol) of diketene was added dropwise, and the reaction temperature was controlled at 70°C during the reaction. After the dropwise addition, the temperature was controlled at 75°C and kept for 6 hours to complete the reaction. The crude ester was rectified under negative pressure to obtain 743 g of finished tert-butyl acetoacetate, with a yield of 94.0% and a content of 99.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com