Heat-resistant cysteine protease, and coding genes and applications thereof
A technology of cysteine protease and coding gene, which is applied in the field of enzyme engineering to achieve high-efficiency protein degradation activity and high-efficiency catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Acquisition of Thermostable Cysteine Protease Gene and Its Recombinant Expression
[0034] 1. Extraction of total DNA samples of microbial communities containing thermostable cysteine protease
[0035] A gelatin wastewater high-temperature anaerobic digestion (UASB process) sludge sample was quickly frozen in liquid nitrogen after sampling and stored at -80°C for later use. After sludge samples were ground with liquid nitrogen, total DNA was extracted according to the FAST DNA Spin kit for Soil (Qbiogene, USA) kit. DNA samples were detected by agarose gel electrophoresis and ultraviolet spectrophotometer, the concentration was adjusted to 100ng / μL, and stored at -20°C.
[0036] 2. Acquisition of thermostable cysteine protease gene
[0037] Using the total DNA obtained in step 1 as a template, use the primer 1 shown in the sequence table SED ID NO.3 and the primer 2 shown in the sequence table SED ID NO.4 to carry out a PCR reaction to amplify the therm...
Embodiment 2
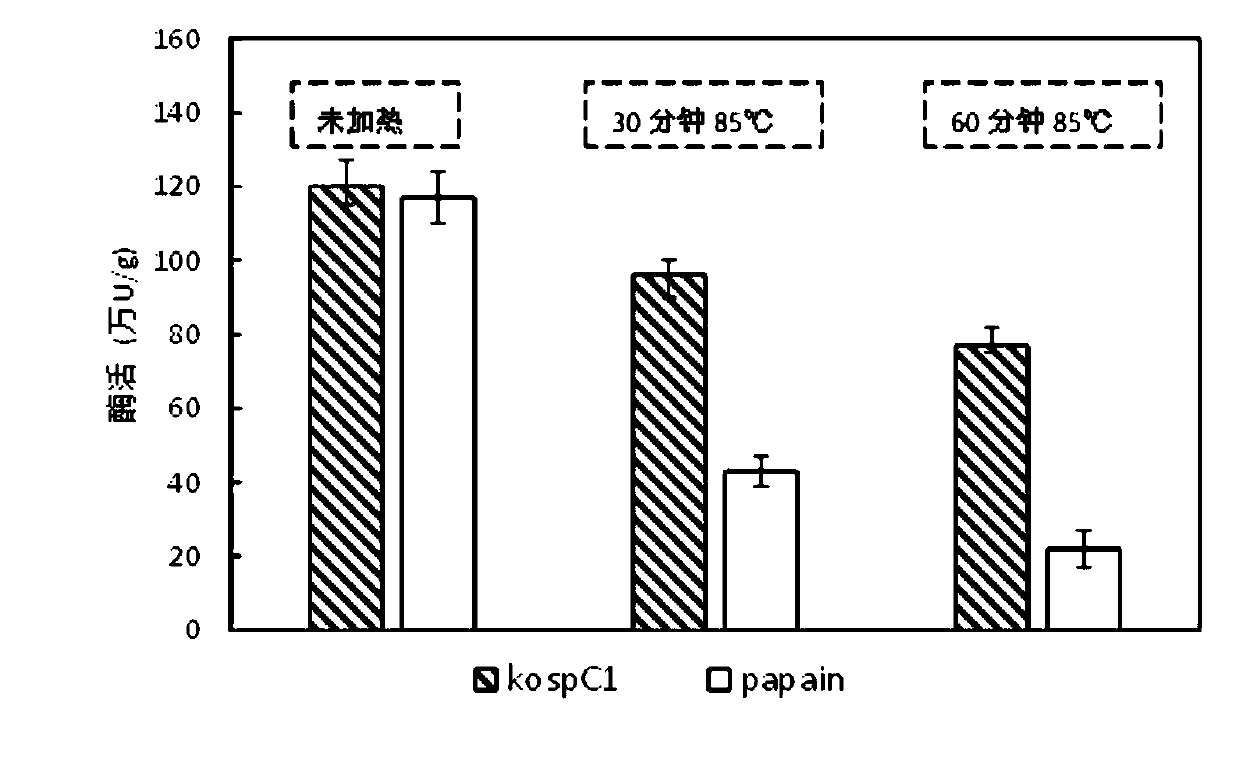
[0046] Example 2 Activity detection of heat-resistant cysteine protease (casein hydrolysis method)
[0047] 1. Principle:
[0048] The target substrate casein is hydrolyzed by cysteine protease under suitable buffer conditions to release free tyrosine or tyrosine-containing small peptides into the reaction solution. After that, trichloroacetic acid was added to the reaction system to precipitate macromolecular proteins (including cysteine protease and unreacted substrate casein), and the tyrosine content in the supernatant was determined by the Folin method, which can indirectly reflect cysteine Definition of the activity level of acid protease: 1 μg of tyrosine produced by hydrolysis of casein per minute at 75°C is defined as 1 unit of protease activity (1U).
[0049] 2. Experimental methods and results:
[0050] 2.1 Test samples
[0051] Cysteine protease kospC1 (derived from Example 1); commercially available papain. Before the test, the dry protease powder was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com