Anari series mongolian drug intragastric floating sustained release preparation and preparation method thereof
A technology of floating and sustained-release preparations in the stomach, applied in the directions of pharmaceutical formulations, digestive system, pill delivery, etc., can solve the problems of reduced patient compliance, easy moisture absorption of powder, loss of drug efficacy, etc., to improve bioavailability, appearance, etc. Smooth and uniform, reducing adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
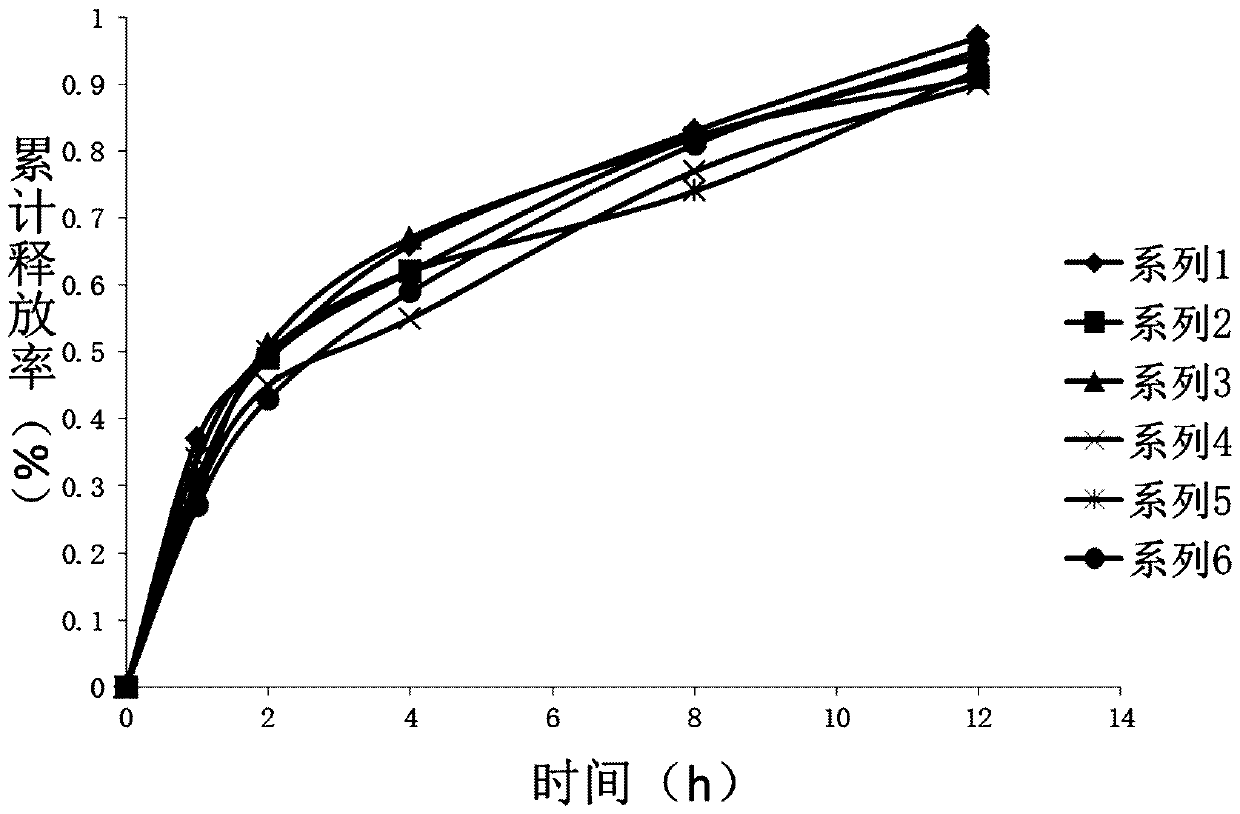
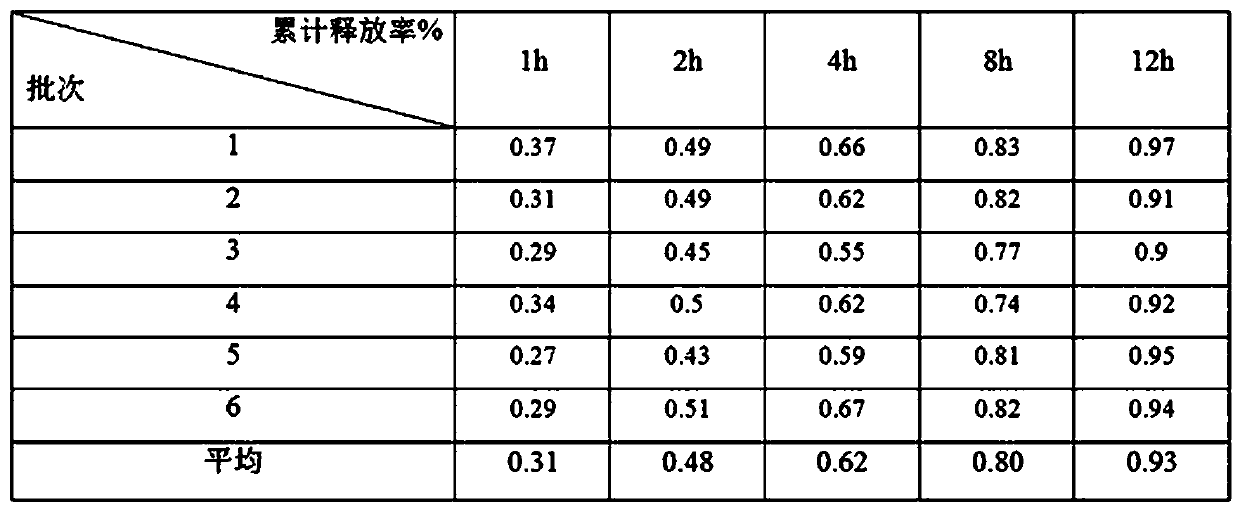
Image
Examples
Embodiment 1
[0040] Embodiment 1: the preparation of Anari-4 extract
[0041] Take 100g of pomegranate, 100g of cinnamon bark, 100g of white cardamom, and 100g of Piper piscifolia as the prescription medicinal materials and grind them, add 8-12 times the volume of 50%-90% ethanol, heat and reflux at 80°C-100°C for 3 times, each time for 60-90min , the extract was cooled to room temperature, suction filtered and combined, and the ethanol was recovered by rotary evaporation until there was no alcohol smell, concentrated to a clear paste with a density of 1.10, freeze-dried for 12-24 hours, and the dried extract was obtained, which was crushed through a 100-mesh sieve to obtain Ana Day-4 extract powder.
[0042] The preparation method of the following Anari series is exactly the same as that of the above Anari-4.
Embodiment 2
[0043] Embodiment 2: the preparation of Anari-5 extract
[0044] Take 250g of pomegranate, 15g of cinnamon, 150g of white cardamom, 100g of longan and 100g of dried ginger and grind them into pieces, add 8-12 times the volume of 50%-90% ethanol, heat and reflux at 80°C-100°C for 3 times, each time After 60-90 minutes, the extract was cooled to room temperature, suction filtered, combined, and the ethanol was recovered by rotary evaporation until it had no alcohol smell, concentrated to a clear paste with a density of 1.10, freeze-dried for 12-24 hours, and the dried extract was obtained, crushed through a 100-mesh sieve, Vested Anari-5 extract powder.
Embodiment 3
[0045] Embodiment 3: the preparation of Anari-6 extract
[0046] Take 100g of pomegranate, 100g of cinnamon, 100g of white cardamom, 100g of longan, 100g of dried ginger, and 100g of bright salt, which are prescribed medicines, and grind them, add 8-12 times the volume of 50%-90% ethanol, and heat and reflux at 80°C-100°C for extraction3 Each time, 60-90min each time, the extract was cooled to room temperature, suction filtered, combined, and the ethanol was recovered by rotary evaporation until there was no alcohol smell, concentrated to a clear paste with a density of 1.10, freeze-dried for 12-24h, and the dried extract was obtained, crushed 100-mesh sieve, acquired Anari-6 extract powder.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| cumulative release rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com